Synthesis of Bis-Dibenzonaphthyridines and Evaluation of their Antibacterial Activity
M. Sangeetha1, M. Manoj2, R. Jayabalan3 and V. Venkateswaran1*
1Erode Arts and Science College, Erode-638 009, Tamil Nadu, India 2College of Pharmacy and Institute of Drug Research and Development, Chungnam National University, Daejeon 305-764, Korea 3Department of Life Sciences, National Institute of Technology, Rourkela, Odhisa, India
DOI : http://dx.doi.org/10.13005/ojc/310227
Article Received on :
Article Accepted on :
Article Published : 29 Apr 2015
Reaction of phthalic acid with 2-methyl-4-N-phenylaminoquinoline under PPA condition resulted in the formation of 6-methyldibenzo[b,h][1,6]naphthyridines, whereas the same reaction with 2,4-bis(N-phenylamino)quinoline resulted in the dimeric dibenzo[b,h][1,6]naphthyridines. A novel mechanism has been proposed to explain the formation of the unexpected product. Screening for the antibacterial activity against various pathogens, proved that the dimeric analogs showed a better antibacterial activity when compared to their monomeric analogs.
KEYWORDS:Chloroquinolines; N-phenylaminoquinolines; dibenzo[b,h][1;6]naphthyridines; dimeric analog; antibacterial activity.
Download this article as:| Copy the following to cite this article: M. Sangeetha M, Manoj M, Jayabalan R, Venkateswaran V. Synthesis of Bis-Dibenzonaphthyridines and Evaluation of their Antibacterial Activity. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: M. Sangeetha M, Manoj M, Jayabalan R, Venkateswaran V. Synthesis of Bis-Dibenzonaphthyridines and Evaluation of their Antibacterial Activity. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8658 |
Introduction
Dimeric chemistry has a wide occurring impact in variety of fields and also proven to be important in the field of drug discovery.1,2 Some of the dimers are found to be equally or even more active with respect to their monomer derivatives. For examples, Indol-3-carbinol3 found in cruciferous vegetables is a naturally occurring modulator of carcinogenesis with a biological activity that is dependent on its conversion of its dimer, i.e., diindolylmethane4 in stomach during digestion. Further exploration on this dimer owing to the importance of fused aromatic ring resulted in a novel anticancer agent.5 Similarly, synthetic bis-indoles posses enhanced cytotoxic activity than their monomers.6 Aaptamine, a naphthyridine alkaloid isolated from marine sponge Aaptos aaptos was found to possess cancer cell growth inhibitory activity7 while its dimer bis-aaptamine, isolated from the marine sponge Aaptos suberitoides showed potent cytotoxic activity against P388 cell lines with IC50 value of 1.8µM.8 Reports on the replacement of chloroquine by its dimeric piperaquine and utilizing it for the treatment of malarial disease caused by Plasmodium falciparum,9 are available. Naturally occurring dimers are of vast biological importance and hence much attention has been paid on their synthesis. Diospyrin, a naturally occurring naphthoquinone dimer isolated from African plant Euclea species has been found to be active against tuberculosis10 and possess good tumor inhibitory effect against different cancer cell lines.11 2,7-Diamino-1,8-naphthyridine dimers have been synthesised and used for the detection of mismatches formed by DNA bases and they also exert therapeutic effects that include antimicrobial, anticancer and antiviral activities.12 Naphthyridine substituted binaphthyl receptors are designed, synthesized and utilised for the fluorimetric discrimination of maleic acid and fumaric acids.13 1,8-Naphthyridines serve as bridging ligands with metal complexes which showed improved photophysical and electrochemical properties.14 Apart from these, many fused dimeric heterocycles from simple scaffolds like thiadiazole based oligomers to fullerene dimers have been synthesised.15-18
With this background and understanding the specific importance of the dimeric heterocycles, particularly naphthyridine analogs, an attempt has been made in this study to synthesize dibenzonaphthyrdine dimers starting from chloroquinoline through arylaminoquinoline, the potential intermediate.
In order to prepare the dimeric dibenzonaphthyridines, the potential intermediate, 2,4ʹ-dimethyl-4-(N-phenylamino)quinoline (1) was first prepared by the reaction of 4-chloro-2-methylquinoline with p-toludine under neat conditions at 160ºC. It was reported earlier that the reaction of 4-arylaminoquinoline with various acids yield the corresponding dibenzo[b,h][1,6]naphthyridines.19,20 Following the same procedure, in the present work, the resulting 2,4ʹ-dimethyl-4-(N-phenylamino)quinoline (1) was allowed to react with phthalic acid expecting to get the bis dibenzo[b,h][1,6]naphthyridines (Scheme 1). The reaction proceeded at 200ºC and the appreance of the C7-singlet in the 1H-NMR confirmed the product obtained as unsubstituted 6,9-dimethyldibenzo[b,h][1,6]naphthyridine (2) instead of the expected dimeric analog. This was further confirmed by the formation of the same product when the intermediate 1 was subjected to Vilsmeier-Haack cyclisation (DMF/POCl3).
In another set of reaction, the intermediate 1 was subjected to cyclisation with piperonylic acid under similar acidic condition. The reaction proceeded at 160ºC to yield two products. The first eluted product after characterization was visualized as 7-(3,4-dihydroxy)substituted-6,9-dimethyldibenzo[b,h][1,6]naphthyridine (3, minor product) and not the expected, i.e., 7-piperonyl substituted-6,9-dimethyldibenzo[b,h][1,6]naphthyridine, whereas the second product was 6,9-dimethyldibenzo[b,h][1,6]naphthyridine (2, major product). It is quite reasonable that the ether bond is broken under acidic conditions at elevated temperatures, but the formation of product 2 was quite interesting. Out of curiosity the intermediate 1 was allowed to react with salicylic acid. The reaction again yielded two products, the major being the product 2, while the minor fraction was the expected 7-(2-hydroxyphenyl) substituted-6-methyldibenzo[b,h][1,6]naphthyridine (4). In both the reactions, the expected products were obtained in poor yields whereas the product 2 (Scheme 1) was obtained in good yield.
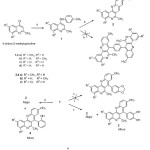 |
Scheme1: Preparation of 6,9-dimethyldibenzo[b,h][1,6]naphthyridine (2) and 7-(hydroxyphenyl) substituted-6-methyldibenzo[b,h][1,6]naphthyridine (3,4)
|
Reagents and Condition
(a) p-Toluidine, Neat condition, 160ºC, 30 minutes, (b) Terephthalic acid, polyphosphoric acid, 200ºC, 5h, (c) Piperonylic acid, polyphosphoric acid, 160ºC, 5h, (d) Salicylic acid, polyphosphoric acid, 160ºC, 5h, (e) DMF, POCl3, 100ºC, 10h.
The elimination of the phenyl group along with carboxyl function can be explained as shown in Scheme 2. As reported by Manoj et al.,21 the reaction gets initiated by C3-acylation of the potential intermediate 1, followed by electrophilic substitution and subsequent aromatization to give the intermediate III via the intermediates I and II. Simultaneously, the carboxyl group gets coupled with the PPA and gets eliminated. The intermediate III is then believed to lose OH and the carboxyl substituted phenyl moieties to afford the reactive carbene intermediate IV which gets converted into zwitter ion V due to the mesomeric effect involving the lone pair of electrons available on the quinoline nitrogen and subsequent migration of proton yields the major product 2. For the hydroxyl substituted carboxylic acids, Iʹ and IIʹ could be the possible intermediates.
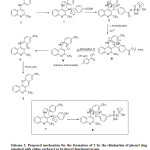 |
Scheme2: Proposed mechanism for the formation of 2 by the elimination of phenyl ring attached with either carboxyl or hydroxyl functional group
|
The reaction does not proceed at lower temperature in case of phthalic acid and elimination reactions occur at elevated temperatures. Based on reports on the effects of the activating groups at C2 and C4 position of quinoline, for the C3-acylation with carboxylic acids,21 it was expected that replacement of the methyl group at the C2 position with another activating groups like phenylamino and subjecting to cyclisation with phthalic acid might yield the expected product. With this view the 2,4-dichloroquinoline was allowed to react with p-toludine to get bis-2,4-di(N-phenylamino)quinoline (5). This potential intermediate was then subjected to cyclisation under acidic condition with phthalic acid (Scheme 3). As expected the reaction proceeded at 100ºC to yield the proposed bisdibenzo[b,h][1,6]naphthyridine (6).
![Scheme 3: Preparation of bisdibenzo[b,h][1,6]naphthyridine (6).](http://www.orientjchem.org/wp-content/uploads/2015/04/Vol31_No2_Synt_Sang_Sch3-150x150.jpg) |
Scheme3: Preparation of bisdibenzo[b,h][1,6]naphthyridine (6). Click here to View scheme |
Reagents and Conditions
a) p-Toludine, Neat condition, 160ºC, 30 minutes, b) Terephthalic acid, polyphosphoric acid, 100ºC, 5h.
Antibacterial Activity
The antimicrobial era has reached the point where the emergence of resistant microbes is accelerating while the pace of discovering new drugs is decelerating.22 Few novel chemical entities have been brought to the market during the past decade and most of them are the derivatives of the known compounds. The pharmacological properties of some compounds have been improved, but they can only temporarily overcome the problem of resistance. There is a need for new classes of antimicrobial compounds particularly for infections caused by various bacteria.. Naphthyridine derivatives exhibit a broad spectrum of biological activities and recently their antimicrobial activities have been reported.23,24 Based on this it is expected that the newly synthesized compounds, 2a-d, 3a, 4a and 6a-d also posses antimicrobial activities.
Source of Microorganisms
In order to test the antimicrobial activity the following microorganisms were used. Escherichia coli, Enterococci faecalis, Proteus mirabilis, Methicillin resistant Staphylococcus aureus, Klebsiella pneumoniae , Proteus vulgaris, Staphylococcus aureus, Morganella morgani, Salmonella typhi.
Test for Antimicrobial Activity
The agar well diffusion method was employed for the determination of antimicrobial activities of the compounds.25 All the tests were performed in duplicate and repeated twice. Modal values were selected. The compounds were weighed and dissolved in DMSO (1mg/mL) and were filter sterilized using a 0.45 µm membrane filter. Each pathogenic microorganism was suspended in sterile saline and diluted to ca. 106 colony forming units (cfu/ml). They were swabbed on to the surface of Mueller–Hinton Agar (MHA). The wells (8 mm in diameter) were cut from the agar and 0.1 mL of DMSO solution with compounds was delivered into them. After incubation for 24 h at 37°C, all plates were examined for any zones of growth inhibition, and the diameter of these zones were measured in millimeters. Amoxicillin and Ceftriaxone (1 mg/1 mL in DMSO) were used as standards. The solvent DMSO was used as negative control.
Results of antibacterial activity of the compounds and standards were summarized in Table 1 and the diameter of the zone of inhibition for few compounds and control against Proteus mirabilis are highlighted in Figure 1.
Table 1. Results of anti-bacterial activity of the compounds and standard
| Compound |
Zone of inhibition in (mm) |
||||||||
| E.coli | Enterococcifaecalis | Proteus mirabilis | Methicillin resistant Staphylococcus aureus | Klebsiellapneumoniae | Proteus vulgaris | Staphylococcus aureus | Morganella morgani | Salmonella typhi | |
|
2a
|
0 |
0 |
8 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2b |
0 |
0 |
0 |
0 |
0 |
0 |
13 |
|
0 |
|
2c |
0 |
0 |
17 |
|
0 |
11 |
18 |
0 |
0 |
|
2d |
0 |
0 |
0 |
0 |
0 |
0 |
13 |
|
0 |
|
3a |
0 |
0 |
12 |
0 |
0 |
0 |
14 |
0 |
0 |
|
4a |
0 |
0 |
14 |
0 |
0 |
10 |
11 |
0 |
0 |
|
6a |
16 |
15 |
21 |
0 |
0 |
18 |
23 |
0 |
0 |
|
6b |
12 |
15 |
8 |
0 |
0 |
0 |
10 |
0 |
0 |
|
6c |
18 |
23 |
28 |
0 |
|
17 |
25 |
0 |
0 |
|
6d |
0 |
16 |
14 |
0 |
0 |
10 |
15 |
0 |
0 |
| Amoxicillin |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
| Ceftriaxone |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
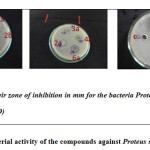 |
Figure1: Antibacterial activity of the compounds against Proteus mirabilis Click here to View figure |
The results in the Table 1 demonstrate that all the dibenzonaphthyridine derivatives have antibacterial activity against the 3 pathogens, namely, Proteus mirabilis, Proteus vulgaris Staphylococcus aureus. A deep analysis of the data reveal that the chloro derivative of the monomer 2c, showed moderate activity when compared to the methyl derivatives 2a and 2b. The hydroxyl derivatives 3a and 4a showed moderate activity against the same pathogens. The interesting results are the dimeric dibenzonaphthyridines 6a, 6b, 6c and 6d showed good activity not only against Proteus mirabilis, Proteus vulgaris, Staphylococcus aureus, but also against E.coli and Enterococci faecalis. The chloro derivative 6c showed excellent antibacterial activity against 5 pathogens and was found to be the best among the compounds synthesised. Some of the zone of inhibition of compounds for the bacteria Proteus mirabilis are highlighted in Figure 2.
Conclusion
The reaction between 2,4-bis(N-phenylamino)quinolines and phthalic acid proceeded at 100ºC to yield bisdibenzo[b,h][1,6]naphthyridines 6a-d, as expected, whereas the reaction of 2-methyl-4-N-phenylaminoquinolines with phthalic acid occured only at 200ºC to yield the unexpected monomeric dibenzo[b,h][1,6]naphthyridines 2a-d. The N-phenylamino group at the 2 and 4 positions serve as effective activating groups for the formation of dimers via C3-acylation at lower temperature while the same N-phenylamino group only at 4th position favours the C3-acylation and cylisation reaction at 200ºC along with the elimination of the carboxylic acid group with phenyl ring. It is also proved that the hydroxyl group behaves similarly and favoured the elimination at 160ºC. The bis dibenzonaphthyridine analogs 6a-d prepared were screened for the antibacterial activity along with the monomers 2, 3 and 4 and the results showed that the dimeric analogs were found to posses excellent activity against various strains tested.
General Experiment Methods
Melting points (m.p) were determined on Mettler FP 51 apparatus (Mettler Instruments, Switzerland) and are uncorrected. They are expressed in degree centigrade (°C). IR spectra were recorded on Schimadzu FTIR-8201PC spectrophotometer (Schimadzu, Japan) using KBr disc. 1H NMR and 13C NMR spectra were recorded on Bruker AMX 400 (400 MHz (1H) and 100 MHz (13C)) and Bruker AV III (500 MHz (1H) and 125 MHz (13C)) NMR spectrometer using tetramethylsilane (TMS) as an internal reference. The chemical shifts are expressed in parts per million (ppm). Mass spectra (MS), were recorded on AutoSpec EI+ shimadzu QP 2010 PLUS GC-MS mass spectrometer. Micro analyses were performed on a Vario EL III model CHNS analyser (Vario, Germany). The purity of the products was tested by TLC with plates coated with silica gel-G with petroleum ether, ethyl acetate and methanol as developing solvents.
Experimental Section
The preparation, spectral and analytical data of the intermediates 2,4′-dimethyl-4-(N-phenylamino)quinoline 1 and 4′,4”-dimethyl-2,4-bis-(N-phenylamino)quinoline 5 are reported in the literature.19-21
Reaction of 1 with phthalic acid; Preparation of 6,9-dimethyldibenzo[b,h][1,6]naphthyridine
To a mixture of 2,4′-dimethyl-4-(N-phenylamino)quinoline (1, 0.002 mol) and phthalic acid (0.0011 mol), polyphosphoric acid (1 g of P2O5 and 0.5 mL H3PO4) was added and heated at 200°C for 5 hours. The reaction mixture showed a single spot on TLC and was poured into ice water and extracted with ethyl acetate, the product was purified using silica gel column chromatography, eluted with a petroleum ether : ethyl acetate (99 : 1) mixture to get 2 as white solid.
2,6,9-Trimethyldibenzo[b,h][1,6]naphthyridine (2a)
White solid; m.p. 222-224 °C; Yield : 35 %; IR (KBr) νmax (cm-1): 1630, 1591 (C=N), 1552, 1448; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.56 (s, 3H, C9-CH3), 2.59 (s, 3H, C2-CH3), 3.02 (s, 3H, C6-CH3), 7.54-7.91 (m, 4H, C3-, C4-, C8-, C10-H), 8.19 (d, 1H, C11-H, J = 8.80 Hz), 8.82 (s, 1H, C7-H), 8.98 (s, 1H, C1-H); 13C NMR (100 MHz, CDCl3) δ (ppm): 17.75, 22.08, 29.78, 117.81, 121.23, 122.45, 126.46, 127.12, 128.41, 128.56, 129.45, 131.34, 133.74 , 134.26, 136.12, 139.24, 147.65, 148.31, 158.75; MS: m/z (%) 272 (M+ 28), 271 (10), 257 (10), 167 (40), 149 (100), 97 (60), 57 (82), 43 (80); Anal. Calcd. for C19H16N2 (272): C ,83.82; H, 5.88; N ,10.30; Found: C, 83.69; H, 6.07; N,10.24 %.
4,6,9-Trimethyldibenzo[b,h][1,6]naphthyridine (2b)
White solid; m.p. 220-222 °C; Yield : 38 %; IR (KBr) νmax (cm-1): 1630, 1600 (C=N), 1530, 1457; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.49 (s, 3H, C9-CH3), 2.73 (s, 3H, C4-CH3), 3.10 (s, 3H, C6-CH3), 7.44-7.97 (m, 4H, C2-, C3-, C8-, C10-H), 8.21 (d, J = 8.51 Hz, 1H, C11-H), 8.89 (s, 1H, C7-H), 9.06 (d, 1H, C1-H, J = 7.44 Hz); 13C NMR (100 MHz, CDCl3) δ (ppm): 17.75, 23.01, 30.04, 117.81, 121.23, 122.45, 126.13, 127.12, 128.16, 128.41, 128.83, 131.34, 133.74, 134.80, 136.14, 139.24, 147.92, 148.39, 158.77; Anal. Calcd. for C19H16N2 (272): C, 83.82; H, 5.88; N, 10.30; Found: C ,83.60; H, 6.05; N, 10.35 %.
2-Chloro-6,9-dimethyldibenzo[b,h][1,6]naphthyridine (2c)
White solid; m.p. 228-230 °C; Yield : 32 %; IR (KBr) νmax (cm-1): 1632, 1598 (C=N), 1537, 1454; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.45 (s, 3H, C9-CH3), 3.06 (s, 3H, C6-CH3), 7.42-8.03 (m, 4H, C3-, C4-, C8-, C10-H), 8.19 (d, 1H, C11-H, J = 8.38 Hz), 8.95 (s, 1H, C7-H), 9.25 (s, 1H, C1-H); Anal. Calcd. for C18H13N2Cl (292): C, 73.97; H, 4.95; N, 7.38; Found: C, 73.35; H, 4.73; N, 7.50 %.
6,9-Dimethyldibenzo[b,h][1,6]naphthyridine (2d)
White solid; m.p. 222-224 °C; Yield : 40 %; IR (KBr) νmax (cm-1): 1633, 1595 (C=N), 1555, 1442; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.60 (s, 3H, C9-CH3), 3.00 (s, 3H, C6-CH3), 7.54-7.91 (m, 5H, C2-, C3-, C4-, C8-, C10-H), 8.22 (d, 1H, C11-H, J = 8.40 Hz), 8.84 (s, 1H, C7-H), 8.99 (d, 1H, J = 8.00 Hz, C1-H); Anal. Calcd. for C18H14N2 (258): C ,83.69; H, 5.47; N ,10.84; Found: C, 83.70; H, 5.47; N,10.83 %.
Preparation of 6,9-dimethyldibenzo[b,h][1,6] naphthyridine (2) from the Vilsmeier-Haack reaction of 1
To a solution of DMF (3.60 mL) at 0°C was added phosphorous oxychloride(8.40 mL) and stirred for 30 minutes. To this reaction mixture was added, 2,4′-dimethyl-4-(N-phenylamino)quinoline 1 (0.001 mol) and heated at 100°C for 10 hrs while monitoring the reaction using TLC. After the completion of the reaction, the reaction mixture was poured into crushed ice and basified with sodium carbonate solution. The reaction mixture was extracted using ethyl acetate. The aqueous layer was once again extracted using ethyl acetate. The combined organic layers were washed with brine solution, dried over anhydrous sodium sulphate, concentrated and purified by column chromatography. Elution with petroleum ether : ethyl acetate (99:1) mixture yielded the product 2.
Reaction of 1 with piperonylic acid: Preparation of 6,9-dimethyldibenzo[b,h][1,6] naphthyridine (2) and 6,9-dimethyl-7-(3′,4′-dihydroxyphenyl)dibenzo[b,h][1,6] naphthyridine 3 – General procedure
To a mixture of 2,4′-dimethyl-4-(N-phenylamino)quinoline (1, 0.001 mol) and piperonylic acid (0.0011 mol), polyphosphoric acid (1 g of P2O5 and 0.5 mL H3PO4) was added and heated at 160°C for 5 hours. The reaction mixture showed two spots on TLC and was poured into ice water, neutralized with saturated sodium bicarbonate solution to remove the excess of piperonylic acid and extracted with ethyl acetate. The two products were separated using silica gel column chromatography eluted with a petroleum ether : ethyl acetate (99 : 1) mixture to get 2 and petroleum ether : ethyl acetate (93 : 7) mixture to get 3 which were recrystallized using ethanol.
2,6,9-Trimethyl-7-(3′,4′-dihydroxyphenyl)dibenzo[b,h][1,6]naphthyridine (3a)
Colourless needles; m.p. 222-224 °C; Yield : 26 %; IR (KBr) νmax (cm-1): 3431, 3360, 1630 ,1591 (C=N), 1552, 1448; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.57 (s, 3 H, C9-CH3), 2.67 (s, 3H, C2-CH3), 3.11 (s, 3H, C6-CH3), 5.29-5.38 (b s, 2H, C3‘, C4‘-OH), 7.27-8.07 (m, 7H, C3-, C4-, C8-, C10-, C2‘-, C5‘-, C6‘-H), 8.18 (d, 1H, C11-H, J = 8.53 Hz), 9.18 (s, 1H, C1-H); 13C NMR (100 MHz, CDCl3) δ (ppm): 20.0, 20.5, 29.8, 118.3, 121.2, 122.4, 125.1, 125.9, 126.3, 126.5, 127.2, 128. 4, 129.7, 130.3, 133.4, 134.8, 136.2, 139.5, 147.9, 148. 0, 148.2 , 148.9, 151.7, 152.4, 159.0; MS: m/z (%) 380 (M+ 100), 379 (20), 366 (10), 355 (15), 286 (17), 272 (22), 256 (35), 136 (100); Anal. Calcd. for C25H20N2O2 (380): C ,78.94; H, 5.26; N, 7.36; Found: C,74.83; H, 6.01; N, 7.96 %.
4,6,9-Trimethyl-7-(3′,4′-dihydroxyphenyl)dibenzo[b,h][1,6]naphthyridine (3b)
White prisms; m.p. 220-222 °C; Yield : 28 %; IR (KBr) νmax (cm-1): 3420, 3380, 1629, 1601 (C=N), 1530, 1457; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.60 (s, 3H, C9-CH3), 2.86 (s, 3H, C4-CH3), 3.08 (s, 3H, C6-CH3), 5.31-5.44 (b s, 2H, C3‘-, C4‘-OH), 7.34-8.05 (m, 7H, C2-, C3-, C8-, C10-, C2‘-, C5‘-, C6‘-H), 8.23 (d, 1H, C11-H, J = 8.70 Hz), 9.15 (d, 1H, C1-H, J = 7.50 Hz); Anal. Calcd. for C25H20N2O2 (380): C, 78.94; H, 5.26; N ,7.36; Found :C, 86.35; H, 5.61; N, 8.04 %.
2-Chloro-6,9-dimethyl-7-(3′,4′-dihydroxyphenyl)dibenzo[b,h][1,6]naphthyridine (3c)
White solid; m.p. 228-230 °C; Yield : 25 %; IR (KBr) νmax (cm-1): 3418, 3388, 1632, 1598 (C=N), 1537, 1454; 1H NMR (400 MHz, CDCl3) δ (ppm): 2.57 (s, 3H, C9-CH3), 3.15 (s, 3H, C6-CH3), 5.35-5.50 (b s, 2H C3‘, C4‘-OH), 7.22-8.22 (m, 7H, C3-, C4-, C8-, C10-, C2‘-, C5‘-, C6‘-H), 8.19 (d, 1H, C11-H, J = 8.38 Hz), 9.14 (s, 1H, C1-H);; Anal. Calcd. for C24H17N2ClO2 (400): C, 72.00; H, 4.25; N, 7.00; Found: C, 78.35; H, 4.73; N, 7.50 %.
Reaction of 1 with salicylic acid: Preparation of 6,9-dimethyldibenzo[b,h][1,6] naphthyridine (2) and 6,9-dimethyl-7-(2′-hydroxyphenyl)dibenzo[b,h][1,6] naphthyridine (4) – General procedure
A mixture of 2,4′-dimethyl-4-(N-phenylamino)quinoline (1, 0.001 mol) and salicylic acid (0.0011 mol) was added to polyphosphoric acid (1 g of P2O5 and 0.5 mL H3PO4) and heated at 160°C for 5 hours. The reaction mixture showed two spots on TLC and was poured into ice water, neutralized with saturated sodium bicarbonate solution to remove the excess of salicylic acid and extracted with ethyl acetate. The two products were separated using silica gel column chromatography and eluted with a petroleum ether : ethyl acetate (99 : 1) mixture to get 2 and petroleum ether : ethyl acetate (94 : 6) mixture to get 4 which were recrystallized using ethanol.
2,6,9-Trimethyl-7-(2′-hydroxyphenyl)dibenzo[b,h][1,6]naphthyridine (4a)
White solid; m. p. 222-224 °C; Yield : 26 %; IR (KBr) νmax (cm-1): 3415 (OH), 1630, 1591 (C=N), 1552, 1448; 1H NMR (500 MHz, CDCl3) δ (ppm): 2.46 (s, 3H, C9-CH3), 2.69 (s, 3H, C2-CH3), 3.09 (s, 3H, C6-CH3), 5.35 (b s, 1H, C2‘ – OH), 7.16-8.06 (m, 8H, C3-, C4-, C8-, C10-, C3‘-, C4‘-, C5‘-, C6‘-H), 8.18 (d, 1H, C11-H, J = 8.53 Hz), 9.03 (s, 1 H, C1-H); 13C NMR (125 MHz, CDCl3) δ (ppm): 19.2, 20.7, 30.0, 118.4, 121.2, 122.5, 126.0, 126.1, 127.3, 128. 2, 128.4, 128.8, 129.0, 129.3, 130.4 , 131.3, 133.3, 134.6, 136.3, 139.2, 147.1, 147.9, 148.4, 150.4, 159.1; MS: m/z (%) 364 (M+ 100, 363 (20), 349 (30), 347 (28), 332 (20), 288 (15), 139 (17), 77 (18), 51 (15); Anal. Calcd. for C25H20N2O (364): C, 82.39; H ,5.49; N, 7.69; Found: C, 82.33; H, 5.41; N, 7.96 %.
4,6,9-Trimethyl-7-(2′-hydroxyphenyl)dibenzo[b,h][1,6]naphthyridine (4b)
White solid; m.p. 220-222 °C; Yield : 25 %; IR (KBr) νmax (cm-1): 3420 (OH), 1628, 1596 (C=N), 1530, 1457; 1H NMR (500 MHz, CDCl3) δ: 2.40 (s, 3H, C9-CH3), 2.59 (s, 3H, C4-CH3), 3.05 (s, 3H, C6-CH3) 5.39 (b s, 1H, C2‘-OH) 7.14-7.90 (m, 8H, C2-, C3-, C8-, C10-, C3‘-, C4‘-, C5‘-, C6‘-H), 8.24 (d, 1H, C11-H, J = 8.22 Hz), 8.95 (d, 1H, C1-H, J = 7.44 Hz); Anal. Calcd. for C25H20N2O (364): C, 82.39; H ,5.49; N, 7.69; Found: C ,82.35; H, 5.61; N, 8.04 %.
2-Chloro-6,9-dimethyl-7-(2′-hydroxyphenyl)dibenzo[b,h][1,6]naphthyridine (4c)
White powder; m.p. 228-230 °C; Yield : 22 %; IR (KBr) νmax (cm-1): 3416 (OH), 1632, 1598 (C=N), 1537, 1454; 1H NMR (500 MHz, CDCl3) δ (ppm): 2.45 (s, 3H, C9-CH3), 3.17 (s, 3H, C6-CH3), 5.44 (b s, 1H, C2‘ – OH), 7.12-7.97 (m, 8H, C3-, C4-, C8-, C10-, C3‘-, C4‘-, C5‘-, C6‘-H), 8.19 (d, 1H, C11-H, J = 8.05 Hz), 9.00 (s, 1H, C1-H); Anal. Calcd. for C24H17N2ClO (384): C, 74.90; H ,4.45; N, 7.28; Cl; 9.21; O, 4.16. Found: C, 75.05; H, 4.43; N, 7.23 %.
Reaction of 4′,4”-dimethyl-2,4-bis-(N-phenylamino)quinoline (5) with phthalic acid: Preparation of 7,7-(1,4-phenylene)bis(9-methyl-N–p-tolyldibenzo[b,h][1,6]naphthyridin-6-amine) (6)
To a mixture of 4′,4”-dimethyl-2,4-bis-(N-phenylamino)quinoline (5, 0.002 mol) and phthalic acid (0.0011 mol), polyphosphoric acid (1 g of P2O5 and 0.5 mL H3PO4) was added and heated at 100°C for 5 hours. The reaction mixture which showed a single spot on TLC was poured into ice water and extracted with ethyl acetate. Purification using silica gel column chromatography and elution with petroleum ether : ethyl acetate (95 : 5) mixture yielded 6 as orange solid.
7,7-(1,4-Phenylene)bis(2,9-dimethyl-N-p-tolyldibenzo[b,h][1,6]naphthyridin-6-amine) 6a
Orange prisms; m.p. >300 οC; Yield: 38 %; IR (KBr) νmax (cm-1): 3423, 1597, 1521, 1346; 1H NMR (500 MHz, CDCl3) δ (ppm): 2.26 (s, 3H, C9-CH3), 2.44 (s, 3H, C4‘-CH3), 2.61 (s, 3H, C2-CH3), 6.95-7.75 (m, 11H, C3-, C4-, C8-, C10-, C2‘-, C3‘-, C5‘-, C6‘-, (C2”,C6”)- and C6-NH), 8.30 (d, 1H, C11-H, J = 8.40 Hz), 8.94 (s, 1H, C1-H); 13C NMR (125 MHz, CDCl3) δ (ppm): 18.5 19.9, 22.2, 112.6, 119.8, 123.1, 123.3, 124.2, 124.6, 125.2, 125.8, 126.6, 128.8, 129.2, 129.5, 129.9, 130.1, 132.1, 133.9, 137.0, 137.3, 144.5, 147.8 , 148.7, 150.4; MS (EI) m/z (%) 800 (M+, 80), 799 (100), 770 (25), 590 (40), 334 (15), 230 (12), 76 (35), 44 (70); Anal. Calcd. for C56H44N6 (800): C, 83.97; H, 5.54; N, 10.49. Found: C, 84.06; H,5.57; N, 10.37 %.
7,7-(1,4-Phenylene)bis(4,9-dimethyl-N-p-tolyldibenzo[b,h][1,6]naphthyridin-6-amine) 6b
Orange prisms; m.p. >300οC; Yield: 40 %; IR (KBr) νmax (cm-1) : 3420, 1596, 1518, 1342; 1H NMR (500 MHz, CDCl3) δ (ppm): 2.31 (s, 3H, C9-CH3), 2.43 (s, 3H, C4‘-CH3), 2.76 (s, 3H, C4-CH3), 7.03-7.75 (m, 11H, C2-, C3-, C8-, C10-, C2‘-, C3‘-, C5‘-, C6‘-, (C2”-, C6”-) and C6-NH), 8.27 (d, 1H, C11-H, J = 8.40 Hz) 8.99 (d, 1H, C1-H, J = 8.00 Hz); 13C NMR (125 MHz, CDCl3) δ (ppm): 18.7, 20.7, 22.0, 111.7, 119.1, 122.4, 122.5, 122.9, 123.3, 123.8, 124.0, 125.1, 126.6, 129.3, 129.7, 129.9, 131.5, 133.6, 133.9, 136.2, 137.4, 144.0, 147.4, 148.9, 149.59; Anal. Calcd. for C56H44N6 (800): C,83.97; H, 5.54; N, 10.49. Found: C, 83.94; H, 5.58; N, 10.48 %.
7,7-(1,4-Phenylene)bis(2-chloro-9-methyl-N-p-tolyldibenzo[b,h][1,6]naphthyridin-6-amine) 6c
Pale yellow spongy mass; m.p. >300οC; Yield: 35 %; IR (KBr) νmax (cm-1): 3413, 1574, 1515, 1321; 1H NMR (500 MHz, CDCl3) δ (ppm): 2.29 (s, 3H, C9-CH3), 2.44 (s, 3H, C4‘-CH3), 7.01-7.71 (m, 11H, C3-, C4-, C8-, C10-, C2‘-, C3‘-, C5‘-, C6‘-, (C2”-, C6”-) and C6-NH), 8.25 (d, 1H, C11-H, J = 8.40 Hz), 9.01 (s, 1H, C1-H); 13C NMR (125 MHz, CDCl3) δ (ppm): 18.8, 20.7, 113.8, 118.6, 122.9, 123.6, 123.9, 124.4, 125.2, 125.8, 126.7, 127.1, 130.0, 130.2, 130.7, 133.1, 133.5, 134.2, 137.8, 138.0, 143.6, 148.0, 149.1, 151.5; Anal. Calcd. for C54H38Cl2N6 (842): C, 77.04; H, 4.55; N, 9.98; Found: C, 77.08; H, 4.48; N, 10.06 %.
7,7-(1,4-Phenylene)bis(9-methyl-N-p-tolyldibenzo[b,h][1,6]naphthyridin-6-amine) 6d
Pale orange prisms; m.p. . >300οC; Yield: 37 %; IR (KBr) νmax (cm-1) : 3416, 1579, 1518, 1337; 1H NMR (500 MHz, CDCl3) δ (ppm): 2.30 (s, 3H, C9-CH3), 2.43 (s, 3H, C4‘-CH3), 7.01-7.78 (m, 12H, C2-, C3-, C4-, C8-, C10-, C2‘-, C3‘-, C5‘-, C6‘-, (C2”-,C6”-) & C6-NH), 8.25 (d, 1H, C11-H, J = 8.40 Hz), 9.07 (d, 1H, C1-H, J = 8.00 Hz); Anal. Calcd. for C54H40N6 (772): C, 83.91; H,5.22; N, 10.87. Found C, 83.80; H, 5.55; N, 10.65 %.
Acknowledgement
The authors thank IISc, Bangalore, IIT, Madras for NMR facilities, Department of Chemistry, Bharathiar University for IR facility and IICT, Hyderabad for Mass facility. The authors thank Dr. Sathish Sankar, Scientist, Sri Sakthi Amma Institute of Biomedical Research, A Unit of Sri Narayani Hospital and Research Centre, Sripuram, Vellore, Tamil Nadu, India for providing clinical isolates.
References
- Gupta, L.; Talwar, A.; Chauhan, P.M. Curr. Med. Chem. 2007, 14(16), 1789-1803.
- Jacobs, M.R.; Bajaksouzian, S.; Good, C.E.; Butler, M.M.; Williams, J.D.; Peet, N.P.; Bowlin, T.L.; Endimiani, A.; Bonomo, R.A. Diagn. Microbiol. Infect. Dis. 2011, 69(1), 114-116.
- (a) Hung, W.C.; Chang, H.C. J. Agric. Food Chem. 2009, 57(1), 76–82 (b) Grose, K.R.; Bjeldanes, L.F. Chem. Res. Toxicol. 1992, 5(2), 188–193.
- Stuab, R.E.; Onisko, B.; Bjeldanes, L.F. Chem. Res. Toxicol. 2006, 19(3), 436–442.
- Chao, W.R.; Yean, D.; Amin, K.; Green, C.; Jong, L. J. Med. Chem. 2007, 50(15), 3412–3415.
- Jiang, B.; Yang C.G.; Xiong, W.N.; Wang, J. Bioorg. Med. Chem. 2001, 9(5), 1149-1154.
- Shaari, K.; Ling, K.C.; Rashid, Z.M.; Jean, T.P.; Abas, F.; Raof, S.M.; Zainal, Z.; Lajis, N.H.; Mohamad, H.; Ali, A.M. Mar. Drugs. 2009, 7(1), 1-8.
- Liu, G.; Tang, X.; Li, P.; Li, G. Org. Lett. 2012, 14(8), 1994-1997.
- Basco, L.K.; Ringwald, P. Antimicrob. Agents chemother. 2003, 47(4), 1391–1394.
- Lall, N.; Sarma, M.D.; Hazra,B.; Meyer, J.J.M. J. Antimicrob. Chemother. 2003, 51, 435–438.
- Ray, S.; Hazra, B.; Mittra, B.; Das, A.; Majumder, H.K. Mol. Pharmacol. 1998, 54(6), 994–999.
- Kobori, A.; Nakatani, K. Bioorg. Med. Chem. 2008, 16(24), 10338–10344.
- Ghosh, K.; Sen, T. J. Phys. Chem. B. 2011, 115(26), 8597–8608.
- Singh, A.N.; Thummel, R.P. Inorg. Chem. 2009, 48(14), 6459–6470.
- Balaji, G.; Shim, W.L.; Parameswaran, M.; Valiyaveettil, S. Org. Lett. 2009, 11(19), 4450-4453.
- Cheng, Y.J.; Chen, C.H.; Ho, Y.J.; Chang, S.W.; Witek, H.A.; Hsu, C.S. Org. Lett. 2011, 13(20), 5484-5487.
- Sisko, J.; Kassick, A.J.; Mellinger, M.; Filan, J.J, Allen, A.; Olsen, M.A. J. Org. Chem. 2000, 65(5), 1516-1524.
- Delgado, J.L.; Osuna, S.; Bouit, P.A.; Martínez-Alvarez, R.; Espíldora, E.; Solà, M.; Martín, N. J. Org. Chem. 2009, 74(21), 8174–8180.
- Manoj, M.; Rajendra Prasad, K.J. Z. Naturforsch. 2009, 64b, 851-857.
- Manoj, M.; Rajendra Prasad, K.J. Synth. Commun. 2012, 42, 434-446.
- Manoj, M.; Rajendra Prasad, K.J. J. Heterocycl. Chem. 2013, 50, 1049-1063.
- Neu, H.C. Science. 1992, 257(5073), 1064–1073.
- Nagate, T.; Kurashige, S.; Mitsuhashi, S. Antimicrob Agents Chemother. 1980, 17(2), 203-208.
- Park, J.K.; Im, S.K,; Ju, H.; Jeon, J.M; Kim, C.G.; Kim, D.U.; Yoo, J.C.; Cho, S.S.; Cho, S.K. Bull. Korean Chem. Soc. 2005, 26, 371-372.
- National Committee for Clinical Laboratory Standards, (NCCLS), Performance Standards for Antimicrobial Disk Susceptibility Test, Approved Standard, 6th edition, Wayne, Pa, Document M2-A6, 1997.

This work is licensed under a Creative Commons Attribution 4.0 International License.









