Synthesis, characterization and biological studies of 1,7- dinaphthyl heptanoids and their metal chelates
S. Sindhu*, Seena Thomachan and V D John
Department of Chemistry, Christ College, Irinjalakuda 680 125, Kerala, India
DOI : http://dx.doi.org/10.13005/ojc/310245
Article Received on :
Article Accepted on :
Article Published : 28 Apr 2015
The present investigation includes the synthesis, characterization, antitumour and antimicrobial activities of three new curcuminoid analogues and their metal chelates. Curcuminoid analogues and their metal chelates have great ability to act as antitumour and antimicrobial agents which motivated us and lead to this present work. The new curcuminoid analogues namely 1,7-di(1-naphthyl)-1,6-heptadiene-3,5-dione (1a), 1,7-di(2-methoxy 1-naphthyl)-1,6-heptadiene-3,5-dione (1b), 1,7-di(2-hydroxy 1-naphthyl)-1,6 -heptadiene-3,5-dione and their Cu(II) and Al(III) complexes were synthesized and characterized using UV, IR,1HNMR and Mass spectral data. The curcuminoid analogues and their metal complexes were studied for their cytotoxicity and antibacterial ability using Trypan blue exclusion method and agar well diffusion method respectively. The present study suggests that the Cu(II) complexs showed remarkable enhancement of cytotoxic activity where as the Al(III) complexs were found to be most active towards antimicrobial activity.
KEYWORDS:1; 7–dinaphthyl heptanoids; NMR; mass spectra; cytotoxicity; antimicrobial activity
Download this article as:| Copy the following to cite this article: Sindhu S, Thomachan S, John V. D. Synthesis, characterization and biological studies of 1,7- dinaphthyl heptanoids and their metal chelates. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Sindhu S, Thomachan S, John V. D. Synthesis, characterization and biological studies of 1,7- dinaphthyl heptanoids and their metal chelates. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8629 |
Introduction
Curcuminoids are bioactive yellow orange pigment present in the rhizomes of the plant, turmeric (Curcuma longa Linn). Turmeric has been used for centuries in Ayurvedic medicine due to its anti- inflammatory, antifungal, antimicrobial and antitumour activities(1-6). Efficiency of synthetic curcuminoids as chemopreventive agents have also been established(7-9). Structurally curcuminoids are linear diarylheptanoids which exist in tautomeric forms as α,β unsaturated 1,3-diketo form and enol form.
Curcuminoid analogues prepared by synthesis retain the α,β unsaturated 1,3-diketo moiety and their metal
chelates possess remarkable biochemical activity(10-11).
In the present work, aldehydes namely 1-naphthaldehyde, 2-methoxy naphthaldehyde and 2-hydroxy naphthaldehyde were condensed with acetylacetone in presence of B2O3 using tri-secondary butyl borate and n-butyl amine as the condensing agent(12). The ligands prepared (Fig.1a, 1b and 1c) were complexed with Cu(II) (Fig.2) and Al(III) to form metal chelates. The curcuminoid analogues and their metal chelates were subjected to in vitro cytotoxic studies using trypan blue exclusion method. These compounds were also subjected to antibacterial activity against the test organisms Escherichia coli, Klebsiella pneumoniae and Bacillus subtilis.
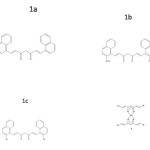 |
Figure 1 Click here to View figure |
Materials and Methods
The chemicals required were obtained from Sigma Aldrich chemical suppliers and are of analar grade. Daltons Lymphoma ascites (DLA) and Ehrilich ascites carcinoma (EAC) cells were obtained from the Cancer Research Institute, Mumbai, India. Bacterial stains namely Escherichia coli, Klebsiella pneumoniae and Bacillus subtilis were obtained from the culture collection of Institute of Microbial Technology (IMTECH), Chandigarh, India.
Analytical Instruments
UV spectra were recorded on a Schimadzu UV-VIS-1601 spectrophotometer. IR spectra (KBr pellets) were recorded on 8101 Schimadzu FTIR spectrophotometer. The 1H NMR spectra were recorded on a Varian 300 NMR spectrophotometer. The FAB mass spectra were recorded on a Joel SX–102 mass spectrophotometer from CDRI, Lucknow, India.
Synthesis of 1,7-Dinaphthyl-1,6-Heptadiene-3,5-Diones and their Derivatives
The curcuminoid analogues were prepared by the condensation of aldehydes (1-naphthaldehyde, 2- methoxy naphthaldehyde and 2-hydroxy naphthaldehyde) with acetylacetone-boric oxide complex in ethyl acetate medium in presence of tributyl borate and n-butyl amine. The products were purified by column chromatography over silica gel (60–120 mesh) using 4:1 (v/v) chloroform:acetone mixture as the eluent and recrystallised twice from hot benzene to get pure crystalline material.
Preparation of Metal Complexes
The Al(III) complexes were prepared by adding a methanolic solution of aluminium nitrate Al(NO3)3.9H2O (25 ml, 0.001mol) to a solution of diketone ( 25 ml, 0.003 mol ) in methanol and refluxed gently for 2 h. After reducing the volume to half, the solution was cooled to room temperature. The precipitated complex was filtered, washed with 1:1, methanol:water mixture and recrystallised from hot methanol. The Cu(II) complexes were prepared by adding a methanolic solution of copper(II) acetate ( 25 ml, 0.001mol ) to a solution of diketone (25 ml, 0.002 mol ) in methanol and the above procedure is repeated.
In vitro Cytotoxicity Study
In vitro cytotoxicity studies were carried out using the diketone, Cu(II) and Al(III) complexes dissolved in minimum quantity of DMSO. The tumour cells aspirated from the peritoneal cavity of tumour bearing mice were washed with PBS (Phosphate buffered saline). Cell viability was determined by trypan blue exclusion method. Viable cells (1×106 cells in 0.1 ml) were added to tubes containing various concentrations of the test compounds and the volume was made up to 1 ml using PBS. Control tube contains only cell suspension. These mixtures were incubated for 3 h at 37oC. Further, cell suspension was mixed with 0.1mol of 1% trypan blue and kept for 2-3 m and loaded on a haemocytometer. The number of stained (dead cells) and unstained (live) cells were counted and percentage cytotoxicity was evaluated by trypan blue exclusion method(13).
Antibacterial Assay (Agar Well Diffusion Method)
Agar plates were prepared using sterile Muller-Hinton (MH) agar medium. Bacterial strains of Escherichia Coli, Klebsiella Pneumoniae and Bacillus Subtilis of 24 h culture were evenly spread into the surface of the agar plates using sterile swab sticks. Wells were cut into agar plates with sterile gel puncture. The curcuminoid analogues and their metal chelates in the concentration 5 mg/ml in DMSO were added in the cells. The pure solvent DMSO act as negative control and streptomycin (5mg/ml) served as positive control. The plates were incubated at 37oC for 24 h and observed zones of inhibition. The antibacterial activity was measured in terms of mean diameter of the zone of inhibition in mm.
Results and Discussion
Structural characterization of 1,7-dinaphthyl-1,6-heptadiene-3,5-diones and their derivatives The compounds prepared were characterized on the basis of UV, IR, 1HNMR and Mass spectral data (Tab. 1). The UV spectra of the compound in methanol show two absorption maxima corresponding to n and transitions. The IR spectra of compounds show a strong band ~ 1640 cm-1 assignable to intra molecularly hydrogen bonded carbonyl function. The 1HNMR spectra of the compounds show peaks due to enol, methine and alkenyl proton. Additionally compound 1b shows a singlet at δ ~4.05 ppm due to methoxy group and 1c shows a peak at δ~10.78 ppm due to ortho hydroxy group. Peaks corresponding to step wise elimination of aryl groups and small fragments are present in the mass spectra.
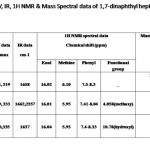 |
Table 1 Click here to View table |
Structural Characterization of Metal Complexes
1,7-Dinaphthyl heptanoids form well defined crystalline complexes with Al(III) and Cu(II) ions. Analytical and mass spectral data (Tab. 2) clearly suggest a ML3 stoichiometry for Al(III) and ML2 for Cu(II) complexes. In the IR spectra of metal chelates, the bond due to intra molecularly hydrogen bonded carbonyl function of the ligand at ~1640 cm-1 disappeared and instead a strong band assignable to stretching of the coordinated carbonyl moiety appeared at ~ 1610 cm-1. Additional bands appear at ~ 475 cm-1 and ~ 420 cm-1 assignable to ν(M–O) vibration. The mass spectra of complexes showed relatively intense peak at m/z corresponding to AlL3 and CuL2, respectively.
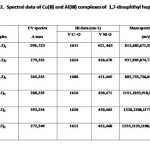 |
Table 2 Click here to View table |
In Vitro Cytotoxicity
The results of In vitro cytotoxicity of the diketones and their Cu(II) and Al(III) complexes towards EAC (Tab. 3) and DLA (Tab. 4) indicate that metal chelation enhance cytotoxicity of compounds considerably. The copper complexes of 1,7-dinaphthyl heptanoids show better results than that of ligands as well as aluminium complexes.
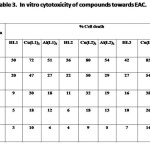 |
Table 3 Click here to View table |
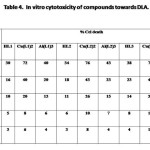 |
Table 4 Click here to View table |
Antibacterial Activity
The results of the antibacterial activity of 1,7-dinaphthyl heptanoids and their complexes (Tab. 5) revealed that the ligands and their complexes possess comparable antibacterial activity to that of standard drug streptomycin. In all the cases, metal complexes possess better antibacterial activity than that of ligands, which means that metal complexation enhance activity. Out of the two metals, aluminium complexes show maximum antibacterial activity.
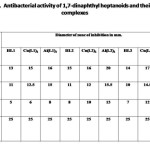 |
Table 5 Click here to View table |
Conclusion
The ongoing discussion reveals that 1,7-dinaphthyl heptanoids and their metal complexes possess enhanced cytotoxicity and antibacterial activity. The metal chelation considerably enhance the cytotoxicity of these compounds. Also it is found that Cu(II) complex of 1c which is a dihydroxy derivative, is the most active compound in in-vitro cytotoxicity studies both with EAC and DLA than Al(III) complexes. The phenolic group together with the β-diketone moiety present in the curcuminoids is suggested to be responsible for their biological activities(14).The substituents on the phenyl ring, especially the hydroxyl groups, play a vital role in deciding the antitumour characteristics of the compounds. Out of the metal complexes, Al(III) complex of 1b is found to be most effective in antibacterial studies.
Acknowledgement
The authors would like to thank the Director, Amala Cancer Research Institute, for the antitumour studies and Dept. of Biotechnology, St. Joseph’s College, Irinjalakuda for antibacterial studies.
References
- Lampe, V. Journal of Milobedzka Bern, 1913, 46, 2335-2339.
- Chandra, D.; Gupta, S. S. Indian Journal of Medical Research, 1972, 60, 138-140.
- Srimal, R.C.; Dhawan, B. N. Journal of Pharmacy and Pharmacology, 1973, 25, 447-452.
- Dheodhar, S. D.; Sethi, R,; Srimal, R. C.; Indian Journal Medical Research, 1980, 71, 632-634.
- Azuine, M .A.; Bhide, S. V.; Nutrition cancer, 1992, 17(1), 77-83.
- Nagabhushan, M.; Bhide, S. V. Journal of American College of Nutrition 1992, 11, 192-198.
- Krishnankutty, K.; Venugopalan, P. Synthesis and Reactivity in Inorganic Metal-Organic Chemistry 1998, 28(8), 1313-1325.
- Krishnankutty, K. John, V. D.; Kuttan, G. Journal of Experimental and Clinical Cancer Research 2002, 21(2), 219-224.
- Krishnankutty, K.; John, V. D. Synhesis and Reactivity in Inorganic Metal-Organic Chemistry 2003, 33(2), 343-358.
- Clare, M. J.; Hydes, D. C., Metal ions in biological systems, metal complexes as anticancer drugs. Marcel Decker, New York (1979).
- Sharma, K. K.; Chandra, S.; Babu, D. K. Inorganica Chimica Acta 1987, 135(1), 47-48.
- Pabon, H. J. J. Recueil des Travaux Chimiques des Pays-Bas 1964, 83, 237-240
- Kuttan, G.; Vasudevan, D. M.; Kuttan, R. Cancer Letters 1988, 41, 307-315
- Kostova, D.; Albena, T.; Paul, T. Carcinogenesis 1999, 20, 911-919 .

This work is licensed under a Creative Commons Attribution 4.0 International License.









