Synthesis and Screening of Fluoro Substituted Pyrazolyl Benzoxazoles
R. K. Jadhav1, A. B. Nikumbh1 and B. K. Karale2*
1P. G. Department of Chemistry, S.S.G.M. College, Kopargaon, Dist. Ahmednagar- 423601, India.
2P. G. Department of Chemistry, Radhabai Kale Mahila Mahavidyalaya, Ahmednagar, 414001, India.
Corresponding Author Email: bkkarale@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310242
Article Received on :
Article Accepted on :
Article Published : 29 May 2015
A series of 3-Formylchromone 1 was reacted with 1-(4-(4-fluorophenyl)thiazol-2-yl)hydrazine 2 to get (1-(4-(4-fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl)(2-hydroxyphenyl) methanone 3 which on reaction with hydroxylamine hydrochloride given methanone oxime 4 and 4 on treatment with POCl3 formed 2-(1-(4-(4-fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl)benzo[d]oxazole 5. The structures of synthesized compounds were confirmed by spectral analysis further they were screened for their biological activity.
KEYWORDS:3-Formylchromones; Methanone; Oxime; Benzoxazole; Spectral analysis; Biological activit
Download this article as:| Copy the following to cite this article: Jadhav R. K, Nikumbh A. B, Karale B. K. Synthesis and Screening of Fluoro Substituted Pyrazolyl Benzoxazoles. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Jadhav R. K, Nikumbh A. B, Karale B. K. Synthesis and Screening of Fluoro Substituted Pyrazolyl Benzoxazoles. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8894 |
Introduction
3-Formylchromones give several versatile condensation reactions as they contain 3-electrophilic centers in the molecule1 which can be converted into various biological active compounds. 3-Formylchromones achieved by the most stable method through the application of Vilsmeir Haack reaction1, 2 from 2-hydroxyacetophenone. It has an instantaneous aldehyde group and undergoes Knovengel condensation reaction and gives several important synthetic compounds. Different researchers were studying the condensation reactions of various nucleophiles with 3-formylchromones. 2-Hydroxyacetophenones are good precursors for several applications, which were synthesized by Fries rearrangement by known procedure3.
Pyrazole nucleus based compounds exhibits focus on medicinal and agriculture chemistry because they found vast scope for biological activities like anticancer4, hepatoprotective5, antidiabetic6, anticancer6, cytotoxic7, herbicidal8 and fungicidal9 activities.
Fluorinated moieties have been found unique properties in synthesizing the compounds in the drug world.
Methanones are effective inhibitors of FLT3-ITD kinase in vitro and stimulate apoptosis in PKC412 sensitive and resistant cell lines10. Many of them are also shown extensive band of bioactivities such as antimycobacterial11, antipsychotic12, antioxidant13, antifeedant13, they are effective inducer of apoptosis14. Oximes are being extensively used as an important intermediate in synthesizing the new heterocycles. Oximes shows acaricidal15, insecticidal16, antiviral (TMV)17 and antimicrobial18, 19 activities. Benzo-fused bicyclic hetero ring structure possess tempting pharmacological activities. Substituted benzoxazole derivatives show anti-HIV20, antitubercular21, anticonvulsant22 and antiproliferative23 activities. Benzoxazole is an antiallergic compound which inhibits the release of mediators of allergic reaction. Benzoxazole also shows two fold 5-Lipoxygenase and Cycloxygenase inhibitors with anti-inflammatory action24.
Due to the extensive applications and biological activities allied with fluorine, pyrazole, benzoxazoles and the utility of oximes as an important intermediate provoked us to synthesize the variously substituted fluorine containing benzoxazole derivatives.
Biological Activities
Antimicrobial Activity
13 synthesized compounds were evaluated for their antibacterial activity against the bacteria E. coli, Pseudomonas aeruginosa and Staphylococcus aureus using standard drugs like Gentamycin and Nystatin. The activities were studied by turbidity method using DMSO as a solvent. The Inhibition zones were measured in mm. The same compounds were also tested for fungal activities against the fungus Candida sp. using the same standard drugs in the same solvent as for antibacterial activities. The used concentration was 1mg/1000mm. At this concentration no activity was observed.
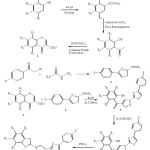 |
Scheme 1 |
Experimental
The Physical constant of all the synthesized compounds were measured in open capillary tubes in paraffin liquid and are uncorrected. Purity of all synthesized compounds was checked by TLC. The IR spectral study was recorded on a Perkin-Elmer (spectrum on a FT-IR) spectrometer. The 1H NMR was studied on a BRUKER AVANCE II 400 MHz NMR Spectrometer in CDCl3 and DMSO as a solvent, chemical shift (δ) were expressed in ppm (scale) downfield from TMS and coupling constant (J) are expressed in hertz (Hz). Mass spectra were recorded on Waters, Q-TOF MICROMASS (LC-MS). TLC was performed on precoated silica plates, which was experiencing under UV illumination. All the synthesized compounds gave an adequate elemental analysis.
(1-(4-(4-Fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl)(2-hydroxyphenyl)methanone
3-Formylchromone (0.02 mole) was dissolved in ethanol with 1-(4-(4-fluorophenyl) thiazol-2-yl) hydrazine (0.02 mole). The reaction mixture was reflux for 30 min to get analogous hydrazone. To the same reaction mixture 22 moles of KOH were added and heating was sustained for a further 6 hr. After finishing point of the reaction the contents were cooled to room temperature and poured into crushed ice. The ensuing solution was neutralized by means of conc. HCl. The solid obtained was separated by filtration and crystallized from ethanol. Compounds synthesized by the above reaction procedure are listed in Table 1.
Table1: Characterization table of synthesized compounds
|
Compound No. |
R1 |
R2 |
R3 |
R4 |
M.P. (0C) |
Yield (%) |
|
3a |
H |
H |
Me |
H |
181-185 |
82 |
|
3b |
H |
H |
H |
H |
130-140 |
75 |
|
3c |
H |
H |
Br |
H |
148 |
79 |
|
3d |
H |
Me |
H |
H |
158 |
69 |
|
3e |
Cl |
H |
Cl |
H |
125-130 |
69 |
|
3f |
H |
H |
Cl |
H |
165-170 |
82 |
|
3g |
H |
Me |
Cl |
H |
190 |
71 |
|
3h |
H |
H |
Et |
H |
104 |
78 |
|
3i |
H |
Me |
H |
Me |
110-112 |
80 |
|
4a |
H |
H |
Me |
H |
220 |
66 |
|
4b |
H |
H |
H |
H |
178 |
68 |
|
4c |
H |
H |
Br |
H |
212-214 |
65 |
|
4d |
H |
Me |
H |
H |
205 |
49 |
|
4e |
Cl |
H |
Cl |
H |
168 |
70 |
|
4f |
H |
H |
Cl |
H |
140-145 |
68 |
|
4g |
H |
Me |
Cl |
H |
200-204 |
45 |
|
4h |
H |
H |
Et |
H |
180 |
58 |
|
5a |
H |
H |
Me |
H |
204-210 |
80 |
|
5b |
H |
H |
H |
H |
180 |
86 |
|
5c |
H |
H |
Br |
H |
185-190 |
90 |
|
5d |
H |
Me |
H |
H |
186 |
89 |
|
5e |
Cl |
H |
Cl |
H |
185 |
68 |
|
5f |
H |
H |
Cl |
H |
240 |
75 |
|
5g |
H |
Me |
Cl |
H |
178-180 |
72 |
|
5h |
H |
H |
Et |
H |
130-140 |
65 |
(3a)
IR (cm-1): 3229 (-OH stretching), 3180 (Ar C-H stretching), 1620 (>C=O stretching), 1538 (-C=N stretching of pyrazole), 656 (C-S stretching); 1H NMR (CDCl3): δ 2.35 (s, 3H), 6.8 (d, 1H, J= 8.6 Hz), 7.0-7.4 (m, 4H), 7.17 (dd, 1H, J= 2.8 Hz), 7.44 (d, 1H, J= 2.8 Hz), 7.6 (s, 1H), 7.8 (s, 1H), 10.2 (s, 1H, -OH proton); MS: m/z 380.05 (M+).
(3b)
IR (cm-1): 3138 (-OH stretching), 3112 (Ar C-H stretching), 1627 (>C=O stretching), 1546 (-C=N stretching of pyrazole), 676 (C-S stretching); 1H NMR (CDCl3): δ 7.02 (m, 2H, J= 8.4 Hz), 7.2 (t, 2H, J= 2 Hz), 7.5 (t, 1H), 7.7 (dd, 1H), 7.9 (s, 1H), 8.05 (m, 2H, J= 2.2 Hz), 8.2 (s, 1H), 8.9 (s, 1H), 10.9 (s, 1H, -OH proton); MS: m/z 364.1 (M+)
(3c)
IR (cm-1): 3282 (-OH stretching), 3189 (Ar C-H stretching), 1640 (>C=O stretching), 1588 (-C=N stretching of pyrazole), 696 (C-S stretching), 548 (C-Br stretching); 1H NMR (DMSO): δ 6.9 (d, 1H), 7.2 (t, 1H, J= 8.8 Hz), 7.56 (d, 1H, J= 2.5 Hz), 7.58 (s, 1H), 7.9 (s, 1H), 7.91-8.0 (m, 2H), 8.2 (s, 1H), 8.9 (s, 1H), 10.6 (s, 1H, -OH proton); MS: m/z 444.88 (M+).
(1-(4-(4-Fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl)(2-hydroxyphenyl)methanone oxime
(1-(2-(4-Fluorophenyl) thiazol-5-yl)-1H-pyrazol-4-yl) (2-hydroxyphenyl) methanone (0.05 mol) was dissolved in 15 mL ethanol. To this 10 mL 40% KOH was added with steady stirring at 100C & equimolar hydroxylamine hydrochloride was added. Further stirring was continued at room temperature for 3-4 hr. Then reaction combination was poured into crushed ice and acidified with acetic acid. The product obtained was separated by filtration and crystallized from ethanol to furnish the pure (1-(2-(4-fluorophenyl) thiazol-5-yl)-1H-pyrazol-4-yl)(2-hydroxyphenyl) methanone oxime. The compounds synthesized by above method are listed in Table 1.
(4a)
IR (cm-1): 3143 (Ar-OH stretching), 3112 (N-OH stretching), 2933 (Ar C-H stretching), 1531 (-C=N stretching), 632 (C-S stretching); 1H NMR (CDCl3): δ 2.2 (s, 3H), 6.8 (d, 1H), 7.0-7.1 (m, 4H), 7.7-7.9 (m, 4H), 8.8 (s, 1H ), 9.6 (s, 1H phenolic –OH proton), 11.7 (s, 1H oxime –N-OH proton); MS: m/z 395.1 (M+).
(4b)
IR (cm-1): 3123 (Ar-OH stretching), 3101 (N-OH stretching), 2911 (Ar C-H stretching), 1551 (-C=N stretching), 623 (C-S stretching); 1H NMR (CDCl3): δ 6.8 (t, 1H), 6.9 (d, 1H), 7.2 to 7.3 (m, 4H), 7.8 (d, 2H, J= 5.2 Hz), 7.9 (m, 2H, J= 5.4Hz), 8.2 (s, 1H), 8.8 (s, 1H, N-OH proton), 9.8 (s, 1H, Ar-OH proton); MS: m/z 371 (M+).
(4c)
IR (cm-1): 3144 (Ar-OH stretching), 3105 (N-OH stretching), 2919 (Ar C-H stretching), 1556 (-C=N stretching), 621 (C-S stretching) 539 (C-Br stretching); 1H NMR (DMSO): δ 6.9 (d, 1H, J= 8.72 Hz),7.3 (t,2H, J= 8.72 Hz), 7.37 (m, 1H), 7.4 (d, 1H, J= 2 Hz), 7.8 (m, 2H), 8.0 (t, 2H, J=8.2 Hz), 8.8 (s, 1H), 10.12 (s, 1H, Ar-OH proton,), 12.06 (s, 1H, N-OH proton). MS: m/z 395.1 (M+).
2-(1-(4-(4-Fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl)benzo[d]oxazole
(1-(4-(4-Fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl)(2-hydroxyphenyl)methanone oxime (0.05 mol) was dissolved in POCl3 (15 mL) and refluxed for 3 hr. Then reaction combination was poured into crushed ice and neutralized the content by adding up sodium acetate, 2-(1-(4-(4-fluorophenyl)thiazol-2-yl)-1H-pyrazol-4-yl) benzo [d] oxazole obtained was separated by filtration, washed carefully with cold stream and crystallized from ethanol to get the clean compounds. The synthesized compounds are listed in Table-1.
(5a)
IR (cm-1): 1636 (Ar –C=C- stretching), 1574 (-C=N stretch of benzoxazole), 1529 (-C=N stretch of pyrazole), 1244 (-C-O-C- stretching), 612 (-C-S stretching); 1H NMR (DMSO): δ 2.4 (s, 3H), 7.10 – 7.49 (m, 6H), 7.85 – 7.88 (m, 2H), 8.32 (s, 1H), 9.04 (s, 1H); MS: m/z 377 (M+).
(5b)
IR (cm-1): 1641(Ar –C=C- stretching), 1593 (-C=N stretch of benzoxazole), 1539 (-C=N stretch of pyrazole), 1233 (-C-O-C- stretching), 608 (-C-S stretching); 1H NMR (DMSO): δ 7.1 (t,2H), 7.3 (m, 3H), 7.5 (m, 1H), 7.7 (m, 1H), 7.8 (dd, 2H), 8.3 (s, 1H), 9.1(s, 1H). MS: m/z: 363 (M+).
(5c)
IR (cm-1): 1642 (Ar –C=C- stretching), 1601 (-C=N stretch of benzoxazole), 1540 (-C=N stretch of pyrazole), 1230 (-C-O-C- stretching), 647 (-C-S stretching), 568 (C-Br stretching); 1H NMR (DMSO): δ 7.3 (t, 2H, J= 8.6Hz), 7.6 (d, 1H), 7.7 (d, 1H, J= 8.6Hz), 8.0 (d, 2H, J= 5.9Hz), 8.1 (t, 2H, J= 5.6Hz), 8.5 (s, 1H), 9.3 (s, 1H). MS: m/z 441 (M+).
Acknowledgement
Authors are gratified to the Principal, S.S.G.M. College, Kopargaon for providing laboratory facilities and constant encouragement for research. Authors also thankful to SAIF, Punjab University for spectral analysis and to Bact-Test Laboratories for biological studies.
References
- Plaskon, A. S.; Grygorenko, O. O.; Ryabukhin, S. V. Tetrahedron. 2012, 68, 2743.
- Ibrahim, M. A.; Ali T. E.; Alnamer, Y. A.; Gabr, Y. A. ARKIVOC. 2010, (i), 98.
- Hocking, M. B. J. Chem. Tech. Biotech. 1980, 30, 626.
- Rostoms, A. F.; Shalaby, M. A.; El Demellawy, M. A. Eur. J. Med. Chem. 2003, 38, 959.
- Habibullah, K.; Shamshir, K.; Mohamed, J. A.; Bahar, A. Bio. & Med. Chem. Letts. 2011, 21(24), 7251.
- Amandeep, k.; Rashmi, A.; Gill, N. S. Int. J. Nat. Prod. Sci. 2012, 1, 247.
- Ahasan, N. B.; Islam, R. M. Bangladesh J. Pharmacol . 2007, i 81.
- Kudo, N.; Furuta, S.; Taniguchi, M.; Endo, T.; Sato, K. Chem. Pharma. Bulletin. 1999, 47(6), 857.
- Zhao, P. L.; Wang, F.; Zhang, M. Z.; et. al. J. Agreec. Foodchem. 2008, 56, 1076.
- Heidel, F.; et. al. British J. Haematology. 2009, 144, 865.
- Dwivedi, N.; Tewari, N.; Tiwari, V. K.; Chaturvedi, V.; Manju, Y. K.; Srivastava, A.; Gaikwad, A.; Sinha, S.; Tripathi, R. P. Bioorg Med Chem Lett. 2005, 15, 4526.
- Dwivedi, N.; Tewari, N.; Tiwari, V. K.; Chaturvedi, V.; Manju, Y. K.; Srivastava, A.; Gaikwad, A.; Sinha, S.; Tripathi, R. P. Bioorg. Med. Chem. Letts. 2005, 15, 4526.
- Thirunarayanan, G. Qscience connect. 2014, 1, 18.
- Jiang, S.; Crogan-Grundy, C.; Drewe, J.; Tseng, B.; Cai, X. S. Bio. and Med. Chem. Letts. 2005,15(20), 4526.
- Dai, H.; Xiao, Y. S.; Liz, Xu X. Y.; Qian, X. H. Chinese Chem. Letts. 2014, 25, 1014.
- Ranatunge, R. R.; Angustyniak, M.; Bandarage, U. K.; et. al. J. Med. Chem. 2004. 47, 2180.
- Ouyang, G.; Chen, Z.; Cai, X.; Song, B.; Bhadury, P. S.; Yang, S.; Jin, L.; Xue, W.; Hu, D.; Zeng, S. Bio. & Med. Chem. 2008, 16(22), 9699.
- Lingappa, M.; Kikkeri, N. M. Can. Chem. Transactions. 2014, 2(3), 343.
- Narwade, S. K.; Karale, B. K.; Jagdhani, S. G.; Chaudhari, C. S.; Rindhe, S. S. Oriental J. Chem. 2008, 24(3), 1029.
- Rida, S. M.; Ashour, F. A.; El-Hawash, S. A.; ElSemary, M. M.; Badr, M. H.; Shalaby, M. A. Eur J Med Chem. 2005,40(9), 949.
- Rana, D. N.; Chhabria M. T.; Shah, N. K.; Brahmkshatriya, P. S. Med. Chem. Research. 2014, 23 (1), 370.
- Bywater, W. G.; Coleman, W. R.; Kamm, O.; Merritt, H. H. J. Am. Chem. Soc., 1945, 67 (6), 905.
- Aiello, S.; Wells, G.; Stone, E. L.; et. al. J. Med. Chem., 2008, 51 (16), 5135.
- Sriniwas, A.; Sagar, J. V.; Sarangapani, M. Int. J. Pharm. Sci. 2010, 2(1), 7.

This work is licensed under a Creative Commons Attribution 4.0 International License.









