Synthesis and Characterization of Polyesteramide urethane derived from Melia Azedarach seed oil
Sageer Ahamad, Ghazala Imran, S. A. Ahmad and A. Hasnat*
Department of Chemistry, G.F. College, Shahjahanpur, U.P. 242001, India hamdarshad@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310271
Article Received on :
Article Accepted on :
Article Published : 28 May 2015
Polyesteramide urethane resin has been synthesized from a nontraditional Malia Azedarach seed oil. The oil converted to N,N-bis (2-hydroxy ethyl) maleia azedrach oil fatty acid (HEMFA) by reacting with diethanol amine. HEMFA reacted with phthalic acid to obtained Malia Azedarach polyesteramide (MAPEA). The MAPEA treated with toluene-2,4-diisocyanate (TDI) in different ratios to synthesized polyesteramide urethane (MAPEAU). The optimum wt-% ratio of the TDI to the MAPEA is selected on the basis fluidity and film properties of the MAPEAU. The synthesized intermediates and final resin have been characterized by FT-IR, 1HNMR spectroscopic analyses and by the measurement of physic-chemical properties like specific gravity, refractive index, acid value, iodine value, soponification value.
KEYWORDS:Malia Azedarach; Vegetable oil; Polyesteramide; Urethane
Download this article as:| Copy the following to cite this article: Ahamad S, Imran G, Ahmad S. A, Hasnat A. Synthesis and Characterization of Polyesteramide urethane derived from Melia Azedarach seed oil. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Ahamad S, Imran G, Ahmad S. A, Hasnat A. Synthesis and Characterization of Polyesteramide urethane derived from Melia Azedarach seed oil. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8841 |
Introduction
Vegetable oils have attracted renewed attention as a raw material for the preparation of commercial polymers1-3. These polymers can offer versatile applications in the field of coatings and adhesive4-7. The utilization of vegetable oils in the manufacture of useful polymer based end product solves not only the problem of waste disposal but it also help in bringing down the cost of the products8,9.
Vegetable oils are triglyceride of different saturated and unsaturated fatty acid has been widely used in making alkyd, polyester, epoxy, polyesteramide resins10. The oil of different seeds like linseed, castor, soyabean, sunflower pongamia glabra etc have been largely used as starting materials in making many valuable polymer10,11. Polyurethane resins as a class are well recognized for their excellent adhesion, flexibility, weather resistance and resistant to chemical and solvents attack12. In addition to these presence of urethane linkages in polyesteramides also make it feasible to cure it at ambient temperature13,14.
Melia azedarach (Bakain) is a moderate sized tree. It is found growing wild in the sub-Himalayan tract upto 1800 meter. Under natural conditions the plants regenerate freely from seeds during the rainy season. The plant has great utility with reference to their wood and largely cultivated by the farmers in rural areas. Melia Azedarach tree also yield non edible seeds which contain about 40 % oil content with sufficient unsaturated fatty acid15. Literature survey reveals that oil of this seed still wait for satisfactory utilization especially in making polymers6-8,14. Keeping these facts in mind in present communication we make an effort to utilized the vegetable seed oil in making polyesteramide urethane.
Experimental
Materials
The seeds of Melia Azedarach were collected from the different places of the shahjahanpur district. The oil was extracted from the dried and crushed seeds through the soxhlet apparatus using petroleum ether as a solvent (60-80o). The fatty acid composition and the results of the physico-chemical characterizations are summarized in the Table 1. Phthallic acid, diethyl ether, methanol were used of analytical grade (Merk-India). Diethanol amine of analytical grade procured from the S.D. Fine chemicals India and was distilled under reduced pressure before use.
 |
Table1: Characterization of MASO, HEMFA, MAPEA Click here to View table |
Syntheses
Synthesis of N,N-bis (2-hydroxy ethyl) Melia Azedarach oil fatty acid (HEMFA)
Diethanol amine 0.007 mol was taken in four necked round bottom flask fitted with an electrical stirrer, thermometer, dropping funnel and condenser. The reaction mixture was heated at 180±5 oC. The Melia azedarach oil (0.1 mol) was added drop wise into the reaction mixture over a period of one hour. The progress of the reaction was monitored by TLC. After the completion of the reaction the product was dissolve in diethyl ether and washed with dilute aqueous sodium chloride solution. The ethereal solution filtered and evaporated in rotatory vacuum evaporator to obtained HEMFA.
Synthesis of Melia Azedarach Polyesteramide (MAPEA)
HEMFA and phthalic acid in equal molar ratio and xylene as a solvent were placed in four necked round bottom flask fitted with a Dean-stark trap thermometer and mechanical stirrer. Reaction mixture was heated up to 200±5 oC. The progress of reaction was monitored by taking the acid value12 at regular intervals. After the completion of reaction the product was taken out from the reaction flask and excess of xylene was remove under reduce pressure to obtain MAPEA.
Synthesis of urethane Modified Polyesteramide (MAPEAU)
Polyesteramide of Melia Azedarach seed oil dissolved in xylene were placed in four necked round bottom flask fitted with Dean-stark trap, thermometer and mechanical stirrer. The toluene-2,4-diisocyanate (TDI) added to reaction mixture in different wt-% ratio of the polyesteramide given in Table 2. The progress of reaction was monitored by the thin layer chromatography and also by the determining the acid values and hydroxyl values at different intervals.
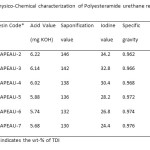 |
Table2: Physico-Chemical characterization of Polyesteramide urethane resins. Click here to View table |
Test Methods
Physico chemical characterizations of HEMFA, MAPEA and MAPEAU were performed as per standard laboratory methods. The structural elucidation was carried out by FT-IR, 1HNMR spectroscopic techniques. The FT-IR spectra of these materials were taken on Perkin Elmer 1750 FT-IR spectrometer (Perkin Elmer Citus instruments Norwalk CT) using NaCl cell. 1HNMR spectra of the resins were recorded on Jeol GSX 300 MHz FX-1000 spectrometer using deuterated chloroform and DMSO as a solvent and tetra methyl silane as an internal standard. The polymeric films were developed on standard strips of 70x25x1 mm size for the bending test.
Results and Discussion
Figure 1(a&b) show the scheme for the preparation of HEMFA, MAPEA and MAPEAU. Significant increase in the hydroxyl value indicates the conversion of triglyceride into diol. The IR spectrum shows the strong band at 3410 cm-1 (broad band for primary alcoholic group). The additional band for carbonyl of amide appears at 1640 cm-1 indicated the formation of amide linkage. The characteristic band for the chain CH2 symmetric and asymmetric stretching appears at 2850-2925 cm-1.
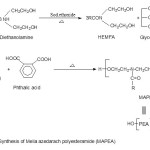 |
Figure 1A Click here to View figure |
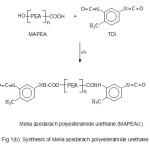 |
Figure 1B Click here to View figure |
The 1HNMR spectrum of HEMFA show the peak at δ=3.5ppm for the CH2 attached to the nitrogen of amide, CH2 attached to hydroxyl group appears at 4.2 ppm. The multiplet peak for proton of double bonded carbon appears at 5.4-5.6 ppm. In addition to these peaks terminal methyl group appears at 0.93 ppm and aliphatic chain CH2 appears at 1.2-1.60 ppm.
The IR spectra of MAPEA shows band at 1740 cm-1 for the carbonyl of ester in addition to band at 1638 cm-1 of the amide confirm the formation of ester linkage. The other characteristic bands like band at 3380 cm-1 appears for alcoholic group, 2921 cm-1 and 2856 cm-1 for asymmetric and symmetric CH2 groups where as the characteristic band for the disubstituted benzene ring appears at 770 cm-1.
The 1HNMR spectrum of MAPEA show the sharp peak of chain –CH2– adjacent to carbonyl of ester at 2.04 ppm, peak for the -CH=CH- at δ= 5.29-5.36 ppm, aromatic proton appears at δ=7.30-7.48 ppm, broad peak for chain –CH2– appears at δ= 1.26-1.30 ppm, terminal –CH3 appears at δ=0.86 ppm.
In the IR spectrum of the MAPEAU a more spread band appears at 3510-3180 cm-1 due to overlap of -OH and -NH groups. In MAPEAU the -NH deformation mode appears at 1557 cm-1. The band for carbonyls of ester appears at 1720 cm-1 where as carbonyl of amide appears at 1648 cm-1. The characteristic band for the benzene ring appears at 1525, 1529, 1545 cm-1.
1HNMR spectrum of the MAPEAU show the terminal methyl group aliphatic chain at δ= 0.9 ppm, where as the methyl group of the TDI appears at δ= 2.1 -2.14ppm. CH2 adjacent to ester appears at δ= 1.5 ppm, CH2 adjacent to amide appears at δ= 1.62 ppm and CH2 adjacent to -C=C- appears at δ= 2.0-2.07 ppm16.
Film Property
The results of the experiment show that on increasing the wt% of TDI in the polymer the drying time decreases however at the same time viscosity of the polymer increases progressively. Therefore more and more solvent required while applying the coating materials. It has been found that after 7-wt % loading of the TDI resulting polymer forms lumpy aggregates and become unbrushable13. This is presumably due to excessive network formation. Furthermore polymeric film obtained by loading of TDI more than 6-wt% does not passes the bending test on 1/8 conical mandrel.
Conclusion
Polyesteramide obtained from nontraditional seed oil Melia azedarach, provides suitable utilization of significant born of the nature. Incorporation of the urethane linkage in the polymer not only improves the performances but also make it curable at room temperature. Preliminary results indicate that urethane obtained by 6-wt % loading of TDI is most suitable polymeric resin for the coating.
References
- Hussain, S. Fawcett, A.H. and Taylor, P. Prog. in Org. coat. 2002.,45 435
- Roy, T.K., Manari, V.M. and Raval, D.A. J. Sci. Ind. Res. 1996., 55 971
- Nayak, P., Mishra, D.K., Parida, D., Shahoo, K. C., Nanda, M., Lenka, S. and Nayak, P. L. J. Appl. Polym. Sci. 1997., 63 671 .,
- Ansrai, S.H., Naseem, M., Hasnat, A. and Ahmad S.A. Biosci. Biotech. Res. Asia 2011.8 ,829
- Kazemi, M. and Dadkhah, A. Orient. J. Chemistry ,2012,.28 .,1201
- Ahmad, S., Naqvi, F., Verma, K.L., Yadav, S. J. Appl. Polym. Sci. 1999.,72 .,1679
- Sharmin, E, Ashraf, S. M., Ahmad S. Eur. J. Lipid Sci. Technol. 2007,. 109 134
- Zafar, F., Ashraf S.M., Ahmad S. J. Appl. Polym. Sci. 2007., 104, 51
- Eren, T., Kusefoglu S. H. J. Appl. Polym. Sci. 2004.,91., 4037
- Raval, D. A., Patel, V. M. Paintindia 2005.,3., 51
- Yeganesh H., Mehdizadeb, H.R., Eur. Polym. J. 2004., 40., 1233
- Yadav S., Zafar, F., Hasnat A., and Ahmad, S. Prog. in Org. coat. 2009.,64., 27
- Sharif Ahmad, Ashraf, S.M., Hasnat A. and Yadav, S. J. Appl. Polym. Sci. 2001.,82., 1855
- Gunduz G. Khalid AH Mecidoglu A. and Aras, L. Prog. in Org. coat. 2004., 49 .,259
- Ambastha, S.P. Useful Plants of India CSIR New Delhi 1986.
- Silverstein, R.M.Bassler G.C. and Morril T.C. spectroscopic Identification of Organic Compounds, Fifth ed, Wiley, New York, 1991.

This work is licensed under a Creative Commons Attribution 4.0 International License.









