Semi-refined κ-carrageenan: Part 1. Chemical modification of semi-refined κ-carrageenan via graft copolymerization method, optimization process and characterization of its superabsorbent hydrogel.
Jamaluddin Mohd. Daud1*, Nor Shuhadah Warzukni2, Rosliza Mohd Salim1 and Ahmad Muzammil Zuberdi2
1Department of Chemistry, Kulliyyah of Science, International Islamic University Malaysia, 25200 Kuantan, Pahang, Malaysia
2Department ofBiotechnology, Kulliyyah of Science, International Islamic University Malaysia, 25200 Kuantan, Pahang, Malaysia
DOI : http://dx.doi.org/10.13005/ojc/310243
Article Received on :
Article Accepted on :
Article Published : 30 Apr 2015
This study was carried out for the preparation of super absorbent polymer hydrogel from semi-refined κ-carrageenan originated from Kappaphycusalvarezii (Doty) Doty ex P. Silva through graft copolymerization method with acrylic acid. The reaction was carried out in an aqueous solution in the presence of N,N-methylene bis-acrylamide as a crosslinker and ammonium persulphate as an initiator. The effect of the amount of cross linker, monomer, initiator and alkali on the swelling capacity of the hydrogel was investigated for optimum conditions.After preparing the desired hydrogel according to optimum conditions, the hydrogel was characterized by FTIR spectroscopy, SEM microscopy and swelling capacity measurement.
KEYWORDS:semi-refined κ-carrageenan; graft copolymerization; optimization; superabsorbent; crosslinking; swelling
Download this article as:| Copy the following to cite this article: Jamaluddin Mohd. Daud J. M, Warzukni N. S, Salim R. M, Zuberdi A. M. Semi-refined κ-carrageenan: Part 1. Chemical modification of semi-refined κ-carrageenan via graft copolymerization method, optimization process and characterization of its super absorbent hydrogel. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Jamaluddin Mohd. Daud J. M, Warzukni N. S, Salim R. M, Zuberdi A. M. Semi-refined κ-carrageenan: Part 1. Chemical modification of semi-refined κ-carrageenan via graft copolymerization method, optimization process and characterization of its super absorbent hydrogel. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8686 |
Introduction
Super absorbent polymers (SAPs) can be defined as three-dimensional networks materials consist of ionic functional groups that can absorb fluids such as water and other aqueous solutions without dissolving into water.1The ability to absorb water is due to the presence of hydrophilic groups such as -OH, -CONH, -CONH2, and -COOH in the hydrogel networks. SAPS can absorb deionized water as high as 1,000-100,000% (10-1000 g/g).2 Hence, they are used in many applications such as personal hygienic products, water retaining in agricultural applications, and drug delivery carriers in medicine.
Currently, most SAPs available in the market are made from synthetic polymers which are petroleum-based. The SAPs are usually non-biodegradable and may give unfavorable impact to the environment and human health. The use of natural polymers such as polysaccharides is always preferred for the production of SAPs because natural polymers are biodegradable, non-toxic and they are available in large quantities from renewable sources. Polysaccharides consist of many functional groups that make them to undergo chemical modification for the purpose of improving their properties for particular applications.3Previous studies have reported the use of graft copolymerization in the formation of natural polymer-based SAPs from alginate4, protein5, starch6, cellulose7and κ-carrageenan8.
κ-carrageenan is a hydrophilic polysaccharide that can be obtained from red seaweeds. It is composed of alternate units of D-galactose-4-sulphate and 3,6-anhydro-D-galactose joined by α-1,3 and β-1,4-glycosidic linkage.9 The schematic diagram of κ-carrageenan chemical structure is shown in Figure 1.It is suitable for the formation of natural-based SAP s because of its structure consists of hydrophilic sulphate groups with high ionization tendency and has low salt sensitivity characteristic.8,10,11The gel strength of κ-carrageenan is very useful in many industrial applications. However, the gelation of κ-carrageenan can be destroyed because it is easy attacked by microorganisms.12Therefore, a chemical modification approach such as the combination of κ-carrageenan with other synthetic monomers is the best way to remove this drawback and to improve their swelling properties in various solutions.
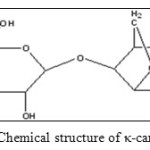 |
Figure1: Chemical structure of κ-carrageenan Click here to View figure |
κ-carrageenan is a relatively new polysaccharide to be used for the preparation of SAP s from natural polymer.13 However, the production of refined κ-carrageenan is very costly and time consuming due to complex purification process during extraction whereas, semi-refined κ-carrageenan from red seaweed is much easier to prepare and cost effective when compared to refined κ-carrageenan. Since there were no previous reports on the application of semi-refinedκ-carrageenan in superabsorbent fields, this study was designed to introduce the material as an ingredient for the preparation of natural-based SAPs hydrogel through graft copolymerization method. The aim of this research was to chemically modify semi-refined κ-carrageenanby crosslinking graft copolymerization of acrylic acid, in the presence of a crosslinker and an initiator. Optimization of grafting conditions was conducted to achieve a semi-refined κ-carrageenan-graft-poly(acrylic acid) (SRκC-g-PAA) hydrogel with swelling capacity as high as possible.
Materials and Methods
Materials
Dried red seaweeds, K. alvarezii (Doty) Doty ex P. Silva was obtained from a seaweed farm in Semporna, Sabah, Malaysia. Acrylic acid (Merck), N,N-methylenebis-acrylamide (Sigma-Aldrich), ammonium persulphate (Merck), sodium hydroxide (Merck) and potassium hydroxide (Merck),were analytical grade reagents and were used without further purification.
Preparation of Semi-Refinedκ-Carrageenan
Dried red seaweed,K. alvarezii (Doty) Doty ex P. Silva was cleaned with distilled water to remove adhering salt, sand and other impurities.The washed seaweed was dried in a laboratory oven at 60 oC. Approximately 120 g of dried seaweeds were weighed and cooked with stirring in 2 L of 10% potassium hydroxide at 80 °C for 3 hours using a water bath.Then, the cooked seaweeds were cooled down at room temperature and the alkaline solution was removed. The product was washed for several times using distilled water to remove the excess potassium hydroxide until the washing became neutral (pH 7), dried in a laboratory oven at 40°C and then ground using a mechanical grinder. The semi-refined κ-carrageenan powder was dried using a freeze dryer to remove any remaining water and stored in an air-tight container to exclude moisture from surroundings prior to further use in experiments.
Preparation of Hydrogel
The preparation of SRκC-g-PAA was carried out through chemical modification of semi-refined κ-carrageenan that was prepared from K. alvarezii (Doty) Doty ex P. Silva,by introducing vinyl monomer on the carrageenan through free-radical graft copolymerization method.The optimization process was performed in order to study the effect of crosslinker, monomer, initiator and alkaline hydrolysis on the swelling behaviour of SRκC-g-PAA.
A known weight of semi-refinedκ-carrageenan was mixed withappropriate volume of distilled water and then placed in a water bath at 80 °C for 20 minutes with stirring until the mixture was homogenized.Then, a certainmolar ratios of N,N-methylenebis-acrylamide (MBA)and acrylic acid (AA)were added together into the mixture, and stirred for 15 minutes. Ammonium persulphate (APS)was then added and the mixture was stirred for 1 hour. Then, the mixture was treated with certain concentrations of sodium hydroxide (NaOH) for alkaline hydrolysis and the solution was stirred for 20 minutes. The wet hydrogel obtained was cut into small pieces and soaked in methanol for 2 hours. Afterwards, the used methanol was removed and the hydrogel was treated with fresh methanol and was soaked for 24 hours. The hydrogel was filtered and dried in a laboratory oven at 40 °C. The dried SRκC-g-PAAhydrogel was ground into powder form and stored in an air-tight container to exclude moisture prior to use.
Materials Characterization
FTIR and SEM Analysis
FTIR spectroscopy (Perkin Elmer) was used to analyze sample of the hydrogel using the attenuated total reflection (ATR) method for sample preparation technique. The IR spectra were plotted as percent (%) transmittance against wave number (in cm-1) in the range of 4000-660 cm-1.The surface morphology of samples was observed by using Scanning Electron Microscopy (SEM) (Carl Zeiss AG).
Swelling Capacity Measurement
In order to determine the optimum quantity of each reagent used that gives the highest water absorbency of SRκC-g-PAA, the equilibrium swelling capacity measurement of SRκC-g-PAA in distilled water has been tested for each parameter. An empty teabag was loaded withSRκC-g-PAA powder (0.10±0.005 g) and immersed in 100 mL of distilled water for 1 hour at room temperature. After that, the teabag was hung for 1 hour in order to remove any excessive water that failed to be absorbed. The swollen gel was then weighed. The equilibrium swelling capacity was measured using Equation 1.8All measurements were replicated three times and the average values were reported.
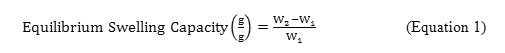
Where W1 is the weight of dried gel in gram and W2 is the weight of swollen gel in gram.
Results and Discussion
Optimization of the Grafting Conditions
Effect of Cross linker
The different mole amounts of MBA were tested in this experiment while the rest of the variables were unchanged.Figure 2 demonstrates the effect of the crosslinker amount on the swelling capacity of SRκC-g-PAA in distilled water.The maximum water absorbency was achieved at 1.95×10-3 moles of MBA with an average reading of water uptake is 256.47 ± 15.07 g/g of the dried hdrogel. The water absorbency decreasing when the amount of crosslinker was too low.This might be due to the hydrogel became soft and fragile when the amount of the crosslinker decreased which resulted in reduced swelling capacity.The water absorbency of SRκC-g-PAA increased when the amount of MBA increased from 1.36×10-3 up to 1.95×10-3 moles because the crosslinker provides spaces in the hydrogel structure for water absorption and holding the hydrogel particles from dissolving in water. Higher than 1.95×10-3 moles, the water absorbency of hydrogel decreased which might be due to the decrease of free space between the copolymer chains when the amount of crosslinker increased. Therefore, the hydrogel cannot expand to absorb more water.8,15 Similar observations have been reported by Pourjavadi et al., (2005).16
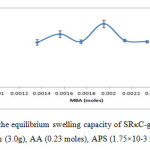 |
Figure2: Effect of crosslinker on the equilibrium swelling capacity of SRκC-g-PAA. Grafting conditions: semi-refined κ-carrageenan (3.0g), AA (0.23 moles), APS (1.75×10-3 moles), NaOH (1.0 N). Click here to View figure |
Effect of Monomer
The mole amounts of AA were varies in this experiment while other conditions were kept constant.The effect of monomer on the equilibrium swelling capacity of SRκC-g-PAA in distilled water are shown in Figure 3.The maximum water absorbency (247.51 ± 14.15 g/g) was achieved at 0.27 moles of AA. The results indicated that the water absorbency of SRκC-g-PAA increased when the amount of AA increased. The increment of water absorption might be due to the network of crosslinked monomer-crosslinker that forms grafted polymers which provide spaces for water molecules to diffuse into the hydrogel. The formation of high carboxylate groups in the SRκC-g-PAA caused a greater affinity for water molecules and enhanced water absorption into the hydrogel particles. However, the water absorbency of the hydrogel decreased with further increase in the amount of AA. This might be due to the homo-polymerization reaction of acrylic acid. Similar observations were reported by Gils et al., (2009).17
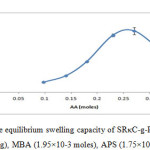 |
Figure3: Effect of monomer on the equilibrium swelling capacity of SRκC-g-PAA. Grafting conditions: semi-refined κ-carrageenan (3.0g), MBA (1.95×10-3 moles), APS (1.75×10-3 moles), NaOH (1.0 N). Click here to View figure |
Effect of Initiator
The mole amounts of APS were varies in this experiment while other conditions were kept constant. The maximum water absorbency (230.86 ± 0.71 g/g) was obtained at 1.10×10-3 moles of APS as shown in Figure 4. The increase in swelling was probably due to the large number of free radicals on the backbone of semi-refined κ-carrageenan. However, the swelling of the hydrogel decreased gradually after the maximum swelling. This might be due to terminating step via bimolecular collision occurred in the presence of higher number of radicals produced that triggered crosslinking density. 18Similar observations was reported in previous research by Sadeghi and Heidari (2011).19
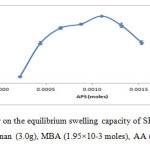 |
Figure4: The effect of initiator on the equilibrium swelling capacity of SRκC-g-PAA. Grafting conditions: semi-refined κ-carrageenan (3.0g), MBA (1.95×10-3 moles), AA (0.27 moles), NaOH (1.0 N). Click here to View figure |
Effect of Alkali
The alkaline hydrolysis of the SRκC-g-PAA was carried out to study the influence of NaOH concentration on the swelling capacity of SRκC-g-PAA. The different concentrations of NaOH were tested, while the rest of the variables were unchanged.As shown in Figure 5, the maximum water absorbency (251.14 ± 15.50 g/g) was obtained at 2.75 N NaOH, at pH 6.This might be due to partial neutralization of SRκC-g-PAA after the addition of NaOH. The increase in the swelling values from 0.50 to 2.75 N was due to anion-anion electrostatic repulsion of the increasing carboxylate anions and osmosis process. In this case, the sodium cations (Na+) from NaOH attracted to the carboxylic acid groups in the hydrogel network and will pull polar water molecules when the hydrogel is placed in water (Nystr and, 2010).20 However, the swelling decreased with the increasing of NaOH concentration can be attributed to the excessive of Na+ remains in the solution after alkaline hydrolysis.These excess ions caused shielding effect phenomenon in which the sodiumcations shield the carboxylate and sulphate anions of the semi-refined κ-carrageenan and consequently prevent anion-anion electrostatic repulsion.21
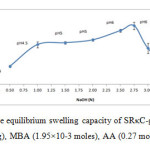 |
Figure5: The effect of alkali on the equilibrium swelling capacity of SRκC-g-PAA. Grafting conditions: semi-refined κ-carrageenan (3.0g), MBA (1.95×10-3 moles), AA (0.27 moles), APS (1.10×10-3 moles). Click here to View figure |
FTIR Analysis
The functional groups and bonds in semi-refined κ-carrageenan were confirmed by comparing the FTIR spectra of the κ-carrageenans product with the commercial semi-refinedκ-carrageenan (Eugene Chemical) and κ-carrageenan primary standard (Sigma) as shown in Figure 6.From the IR spectrum of semi-refined κ-carrageenan prepared from K. alvarezii (Doty) Doty ex P. Silva (Figure 6(c)), the bands observed at 844.14 cm-1, 925.36 cm-1, 1031.05 cm-1 and 1218.83 cm-11 indicated the presence of C–O–SO3 on D-galactose-4-sulphate, C–O of 3,6-anhydro-D-galactose, glycosidic linkage (C–O) of 3,6-anhydro-D-galactose and S=O stretching of sulphate esters, respectively, which were typical features for κ-carrageenan.22 There were similar results with the spectra bands present for the commercial semi-refined κ-carrageenan in Figure 6(b), which were 845.44 cm-1, 926.71 cm-1, 1026.55 cm-1 and 1224.06 cm-1 and in the κ-carrageenan primary standard Figure 6(a), which were 845.00 cm-1, 925.86 cm-1, 1074.55 cm-1 and 1260.00 cm-1. Therefore,it was confirmed that semi-refined κ-carrageenan produced have similar functional groups with those of the commercial semi-refined κ-carrageenan and κ-carrageenan primary standard. Dewi et al., (2012)23 who studied the characteristics and quality of semi-refined κ-carrageenan products based on FTIR spectroscopy showed the spectra bands present were similar with the spectra bands observed by the semi-refined κ-carrageenan prepared in this study.
 |
Figure6: FTIR spectra of κ-carrageenan primary standard (a), commercial semi-refined κ-carrageenan (b), and semi-refined κ-carrageenan product (c). Click here to View figure |
FTIR spectroscopy was also carried out to identify the functional groups of the SRκC-g-PAA hydrogel. The IR spectrum of the SRκC-g-PAA was compared with semi-refined κ-carrageenan and acrylic acid(Figure 7). As compared to the IR spectra of semi-refined κ-carrageenan (Figure 7 (a)) and acrylic acid (Figure 7(b)), the IR spectrum of the SRκC-g-PAA (Figure 7 (c)) showed there were new absorption bands at 1557.68 cm-1, 1452.45 cm-1 and 1403.84 cm-1 after graft copolymerization. These peaks attributed to symmetric and asymmetric stretching mode of carboxylate salts (COO¯Na+). These bands indicated the formation of graft copolymer product.
 |
Figure7: FTIR spectra of semi-refined κ-carrageenan (a), acrylic acid (b) and SRκC-g-PAA (c). Click here to View figure |
SEM Analysis
SEM was used to examine the differences of surface morphology between the semi-refined κ-carrageenan and the SRκC-g-PAA. The surface morphology of the semi-refined κ-carrageenan and SRκC-g-PAA is presented in Figure 8 and 9, respectively.As shown in the SEM images, semi-refined κ-carrageenan has a round porous structure. After the graft copolymerization of acrylic acid onto semi-refined κ-carrageenan, the structural morphology of SRκC-g-PAA was changed. It has a compact structure with smoother and fracture surface morphology. The compact structure confirmed the presence of crosslinking density and grafting network in the SRκC-g-PAA.
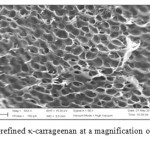 |
Figure8: SEM micrograph of semi-refined κ-carrageenan at a magnification of 633x and the scale bar is 20 µm. Click here to View figure |
 |
Figure9: SEM micrograph of SRκC-g-PAA at a magnification of 1.01 Kx and the scale bar is 10 µm. Click here to View figure |
The presence of fracture surface suggested that the hydrogel possess a porous structure in which the pores were supposed to be the regions available for water retention. The water molecules from external solution were moved into the fracture structure and trapped inside the hydrogel because the small pores restrict them from leaving the three-dimensional network. Because of crosslinking, the SRκC-g-PAA has pores that are smaller in size compared to the semi-refined κ-carrageenan.
Conclusion
In this present study, a superabsorbent hydrogel, SRκC-g-PAA hydrogel was successfully prepared by crosslink graft copolymerization of acrylic acid onto semi-refined κ-carrageenan in the presence of MBAas a crosslinking agent and APS as an initiator K. alvarezii (Doty) Doty ex P. Silva was treated with alkaline solution to produce semi-refined κ-carrageenan, which is cheap and easy to prepare by involving single step preparation from the seaweed. The effect of the amount of crosslinker, monomer, initiator and alkali onto semi-refined κ-carrageenan was performed to achieve the maximum grafting parameters.The maximum absorbency in water was achieved under optimum conditions that were found to be 1.95×10-3 moles of MBA, 0.27 moles of AA, 1.10×10-3 moles of APS and 2.75 N NaOH, for every 3.0 g of semi-refined κ-carrageenan. Identification of the grafting and functional groups of the hydrogel using FTIR spectroscopy showed the presence of bands at 1403.84 cm-1, 1452.45 cm-1 and 1557.68 cm-1 that attributed to symmetric and asymmetric stretching mode of carboxylate salts (COOˉ Na+). These bands were verifying the formation of graft copolymer product. Based on SEM analysis, it was found that the SRκC-g-PAA exhibited a compact structure with fracture surface that indicated the presence of crosslinking density and spaces for water diffusion.
Acknowledgements
The authors would like to express greatest appreciation to The Ministry of Higher Education (MOHE) of Malaysia for the financial support of this study through the ERGS grant and to the International Islamic University of Malaysia (IIUM) for the provision of the research facilities. We also thank to all Kulliyyah of Science staffs for technical supports during this work.
References
- Kiatkamjornwong, S. Science Asia 2007, 33, 39-43
- Zohuriaan-Mehr, M. J.; Kabiri, K. Iran. Polym. J. 2008, 17, 451-477
- Salisu, A.; Naim, A. A.; Sanagi, M. M. IOSR J. Appl. Chem.2013, 4, 39-44
- Pourjavadi, A.; Amini-Fazl, M. S.; Hosseinzadeh, H.Macromol. Res. 2005, 13, 45-53
- Pourjavadi, A.; Kurdtabar, M.; Mahdavinia, G. R.; Hosseinzadeh, H. Polym. Bull.2006, 57, 813-824
- Lanthong, P.; Nuisin, R.; Kiatkamjornwong, S. Carbohydr Polym.2006, 66, 229-245
- Thakur, V. K.; Thakur, M. K.; Gupta, R. K. Carbohydr Polym.2013, 97, 18-25
- Pourjavadi, A.; Harzandi, A. M.; Hosseinzadeh, H. Eur. Polym. J. 2004, 40, 1363-1370
- Dafader, N. C.; Ganguli, S.; Sattar, M. A.; Haque, M. E.; Akhtar, F. Malaysian Polym. J. 2009,4,37-45
- Hosseinzadeh, H.; Sadeghzadeh, M.; Babazadeh, M. J Biomater Nanobiotechnol. 2011, 2, 311-317
- Sadeghi, M.; Ghasemi, N.; Kazemi, M.; Soleimani, F. Middle-East J. Sci. Res. 2012, 11, 311-317
- Srivastava, A.; Kumar, R. Int. J. Carbohyd. Chem. 2013, 2013, 1-8.
- Pourjavadi, A.; Harzandi, A. M.; Hosseinzadeh, H. Macromol. Res. 2005, 13, 483-490
- Pourjavadi, A.; Hosseinzadeh, H. Bull. Korean Chem. Soc.2010, 31, 3163-3172
- Sadeghi, M.; Yarahmadi, M. J. Chem. Chem. Eng. 2011,5, 569-575
- Pourjavadi, A.; Amini-Fazl, M. S.; Hosseinzadeh, H. Macromol. Res. 2005,13, 45-53
- Gils, P. S.; Ray, D.; Mohanta, G. P.; Manavalan, R.; Sahoo, P. K. Int J Pharm Pharm Sci.2009,1,43-54
- Sadeghi, M.; Yarahmadi, M. Afr. J. Biotechnol.2011,10,12265-12275
- Sadeghi, M.; Heidari, B. Materials2011, 4, 543-552
- Nystrand, C. Feasibility of lignocellulose as feedstock for biological production of super absorbent polymers. Master’s Thesis, Linkoping University, Sweden 2010
- Hosseinzadeh, H.; Pourjavavdi, A.; Zohuriaan-Mehr, M. J. J. Biomater. Sci. Polymer Edn.2004,15, 1499-1511
- Mehta, A. S.; Mody, K. H.; Iyer, A.; Ghosh, P. K. Indian J. Chem. Technol.2008, 15, 45-52
- Dewi, E. N.; Darmanto, Y. S.; Ambariyanto. J. Coast.Dev. 2012, 16, 25-31

This work is licensed under a Creative Commons Attribution 4.0 International License.









