Preparation of 1,1-diacetates from aldehydes by LiBH4 and Ac2O in the presence of cation exchange resin
Reza Rezaee Khordehforosh, Behrooz Khezri and Davood Setamdideh*
Department of Chemistry, Collegeof Sciences, Mahabad Branch, Islamic Azad University, Mahabad, Iran.
Correspoding Author
E-mail: davood.setamdideh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310278
Article Received on :
Article Accepted on :
Article Published : 19 Jun 2015
A variety of 1,1-diacetates have been produced from the corresponding aldehydes (1 mmol) by LiBH4(1.25 mmol) and Ac2O (1 mL) in the presence of DOWEX(R)50WX4 (0.5 g) as a cation exchange resin within 10 min at room temperature with excellent yields of the products (93-97%).
KEYWORDS:LiBH4; cation exchange resin; acylal; gem-diacetate; Ac2O
Download this article as:| Copy the following to cite this article: Khordehforosh R. R, Khezri B, Setamdideh D. Preparation of 1,1-diacetates from aldehydes by LiBH4 and Ac2O in the presence of cation exchange resin. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Khordehforosh R. R, Khezri B, Setamdideh D. Preparation of 1,1-diacetates from aldehydes by LiBH4 and Ac2O in the presence of cation exchange resin. Available from: http://www.orientjchem.org/?p=9349 |
Introduction
Carbonyl functional group of aldehydes has been protected in organic synthesis by the preparation of 1,1-diacetates(gem-diacetates, acylals) from the corresponding aldehydes 1-2because 1,1-diacetates are stable under neutral, basic and critically controlled acidic conditions. Also, these compounds have been used as starting materials for Diels-Alder reactions 3, Grignard 4a,Barbier4b,Prins reactions 5,Knoevenagel6a and benzoin condensation. 6b1,1-diacetates are also used in the synthesis of chrysanthemic acid7a,sphingofungins E & F7b and were utilized as cross linking reagents in cotton2. Severalmethods8-50 have been employed for the synthesis of 1,1-diacetates. These methods have some disadvantages such as use of strong acids, moisture sensitivity, high toxicity, high cost of the reagents, unavailable catalyst, harsh reaction conditions and long reaction times, So, the research for new methods for the preparation of 1,1-diacetates is still of importance. This subject encourages us to investigate the preparation of 1,1-diacetates from the corresponding of aldehydes in continuation of our research programs51-59. Herein, We now report the results of a parallel investigation for the fast and efficient method for the synthesis a variety of 1,1-diacetates with LiBH4/Ac2O in the presence of DOWEX(R)50WX4 as acation exchange resin.Former, NaBH458 and Zn(BH4)259have been proposed as a co-reactant.
Results and Discussion
DOWEX (cation exchange resin) as catalyst has been used for the synthesis of Oximes, 51 reduction of carbonyl compounds, 52 synthesis of cyanohydrins 53 and reductive-aminationof aldehydes54. In this context, we have used it for efficient and facile preparation of 1,1-diacetates by LiBH4/Ac2O/DOWEX(R)50WX4 at room temperature.
Benzaldehyde as a model compound has been selected. The reaction of benzaldehyde was carried out in different amounts of LiBH4, Ac2O, DOWEX(R)50WX4 and solvents for the selection of proper conditions at room temperature. Different solvents (THF, diethyl ether, CH2Cl2, CH3CN, n-hexane, Ac2O, THF-Ac2O, Ac2O-CH3CN,…) have been tested.We have observed the reaction was most facile and proceeded to give the highest yield in Ac2O. The optimization reaction conditions showed that using 1.25 molar equivalents of LiBH4 and 0.5 g of DOWEX(R)50WX4 in 1 mL Ac2O were the best conditions to complete the reaction of benzaldehye (1 mmol) to 1,1-benzyldiacetate within 10 min with 93% yields of product as shown in scheme 1.
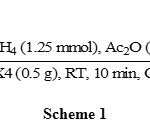 |
Scheme 1 Click here to View scheme |
This protocol was further examined by using various structurally different aldehydes. The corresponding1,1-diacetates were prepared in excellent yields (93-97%) within 10 min as shown in Table 1.
The proposed mechanism for the influence of LiBH4, Ac2O and DOWEX has been shown in scheme II.It is appear SO3H groups on DOWEX (cation exchange resin, strong acid) protonate the carbonyl group of aldehydes (scheme II, step I). So the carbonyl group is more susceptible for Ac2O attack (scheme II, step II).Also, LiBH4 can promote the reaction by the hydride attack (scheme 2, step III).
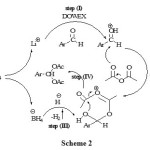 |
Scheme Click here to View scheme |
Cinnamaldehyde as an enal has been converted to the corresponding cinnamylacylal in 95% yield as shown in scheme 3.
 |
Scheme 3 Click here to View scheme |
The C=O stretching frequency in FT-IR spectrum appears around 1738-1763 cm-1. Also, the products were characterized by the 1H-chemical shift of the CH group which appears around 6.8-8 ppm as a singlet (1H) and 1,1-diacetates group which appears around 2.1 ppm as a singlet (6H).
Also, our experiments showed that ketones do not react with this system.
The reusability of the catalyst has been checked by using the recovered DOWEX(R)50WX4 from the reactions. The experiments have shown that the recovered catalyst after regeneration(It was stirred in HCl 10-5% for 30-60 min, and then washed with distillated water) could be used like the first.
Experimental
General
The IR and 1H NMR spectra were recorded on Perkin Elmer FT-IR RXI, 300 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR, 13C NMR or IR spectra and comparison with authentic samples (melting points). All substrates and reagents were purchased from commercially sources with the best quality and used without further purification. All yields referred to isolated pure products.
Table1: The preparation of 1,1-diacetates from aldehydes (1 mmol) by LiBH4 (1.25 mmol),
Ac2O (1 mL) & DOWEX(R)50WX4 (0.5 g) at RT.
|
Table 1. The preparation of 1,1-diacetates from aldehydes (1 mmol) byLiBH4 (1.25mmol), Ac2O (1 mL) &DOWEX(R)50WX4 (0.5 g) at RT. |
||||||
|
Entry |
Aldehydes |
Products |
Time(min) |
Yields(%)a |
m.p.(ºC)b |
|
|
1 |
benzaldehyde |
1,1-diacetoxy-1-phenyl methane |
10 |
93 |
44-45 |
|
|
2 |
4-bromobenzaldehyde |
1,1-diacetoxy-1-(4-bromophenyl) methane |
10 |
97 |
94-95 |
|
|
3 |
2-chloronzaldehyde |
1,1-diacetoxy-1-(2-chlorophenyl) methane |
10 |
95 |
52-53 |
|
|
4 |
4-chlorobenzaldehyde |
1,1-diacetoxy-1-(4-chlorophenyl) methane |
10 |
95 |
83-84 |
|
|
5 |
3-chlorobenzaldehyde |
1,1-diacetoxy-1-(3-chlorophenyl) methane |
10 |
97 |
64-65 |
|
|
6 |
2,4-dichlorobenzaldehyde |
1,1-diacetoxy-1-(2,4-dichlorophenyl) methane |
10 |
97 |
99-101 |
|
|
7 |
4-nitrobenzaldehyde |
1,1-diacetoxy-1-(4-nitrophenyl) methane |
10 |
96 |
124-125 |
|
|
8 |
3-nitrobenzaldehyde |
1,1-diacetoxy-1-(3-nitrophenyl) methane |
10 |
95 |
66-67 |
|
|
9 |
4-methoxybenzaldehyde |
1,1-diacetoxy-1-(4-methoxyphenyl) methane |
10 |
95 |
65-66 |
|
|
10 |
3-methoxybenzaldehyde |
1,1-diacetoxy-1-(3-methoxyphenyl) methane |
10 |
96 |
oil |
|
|
11 |
2-methoxybenzaldehyde |
1,1-diacetoxy-1-(2-methoxyphenyl) methane |
10 |
93 |
69-70 |
|
|
12 |
3,4-dimethoxybenzaldehyde |
1,1-diacetoxy-1-(3,4-dimethoxyphenyl) methane |
10 |
94 |
64-65 |
|
|
13 |
4-methylbenzaldehyde |
1,1-diacetoxy-1-(4-methylphenyl) methane |
10 |
94 |
80-81 |
|
|
14 |
cinnamaldehyde |
1,1-diacetoxy-1-(cinammyl) methane |
10 |
95 |
83-85 |
|
| a Yields refer to isolated pure products. b The melting points were compared with the literature.46-50 | ||||||
Reaction of benzaldehyde with LiBH4/Ac2O/DOWEX(R)50WX4 system, A typical procedure
In a round-bottomed flask (5 mL) equipped with a magnetic stirrer, a mixture of benzaldehyde (0.106 g, 1 mmol), Ac2O (1 mL) and DOWEX(R)50WX4 (0.5 g) was prepared. Then the LiBH4 (0.028 g, 1.25 mmol) was added to the reaction mixture and stirred at room temperature. TLC monitored the progress of the reaction (eluent; n-hexan/EtOAc: 9/1). The reaction was filtered after completion within 10 min. After completion of the reaction, the catalyst was filtered and washed with ethyl acetate (15 mL). The combined organic layers were washed with saturated NaHCO3 solution (3 × 10 mL) and water (10 mL) and then dried over anhydrous Na2SO4. The solvent were removed on a rotary evaporator under reduced pressure to give pure product. 1, 1-diacetoxy-1-phenylmethane has been obtained (0.l 93 g, 93% yield, Table 1, entry 1) which was characterized by 1H-NMR, 13C-NMR and FT-IR spectroscopy. 1H-NMR (CDCl3): δ 2.14 (s, 6H), 7.41-7.43 (Ar, 3H), 7.52-7.55 (Ar, 2H), 7.69 (s, 1H) ppm; 13C-NMR (CDCl3): δ 20.85, 89.67, 126.65, 128.58, 129.74, 135.40, 169.79; IR (KBr) ν = 3021, 1761, 1373, 1243, 1216, 1009, 767 cm−1.
Conclusion
In this context, we have shown that the LiBH4/Ac2O/DOWEX(R)50WX4 system is convenient for the preparation of 1,1-diacetates from aldehydes in high to excellentyields. The reactions were carried out with 1.25 molar equivalents of LiBH4 and Ac2O (1mL) in the presence of 0.5 g DOWEX(R)50WX4 at room temperature. Shorter reaction times, easy work-up procedure makes as an attractive new protocol and it could be a useful addition to the present methodologies.
Acknowledgments
The authors gratefully appreciated the financial support of this work by the research council of Islamic Azad University branch of Mahabad.
References
- (a) Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis, 3nd Ed., Wiley-VCH, New York, 1999; p 306..
- Frick, J. G.; Harper, R. J.; Jr. J. Appl. Polymer Sci.1984, 29, 1433-1447.
- Banks, R. E.; Miller, J. A.; Nunn, M. J.; Stanley, P.; Weakley, T. J. R.; Ullah, Z. J. Chem Soc. Perkin Trans.1.1981, 1096-1102.
- (a) Sandberg, M.; Sydnes, L. K. Tetrahedron Lett.1998, 39, 6361-6364. (b) Sydnes, L. K.; Sandberg, M. Tetrahedron1997, 53, 12679-12690.
- (a) Mowry, D. T. J. Am. Chem. Soc.1950, 72, 2535-2537. (b) Merten, R.; Muller, G. Angew. Chem.1962, 74, 866-871.
- (a) Trost, B. M.; Vercauteran, J. Tetrahedron Lett. 1985, 26, 131-134. (b) Trost, B. M.; Lee, C. B.; Weiss, J. M. J. Am. Chem. Soc. 1995, 117, 7247-7248. (c) Sandberg, M.; Sydnes, L. K. Org. Lett.2000, 2, 687-689.
- (a) Kula, J. Pol. Pat. PL 143: 824,1988; (Chem. Abstr. 112, 216290y). (b) Trost, B. M.; Lee, C. J. Am. Chem. Soc.2001, 123, 12191-12201.
- Reddy, A.V.; Ravinder, K.; Reddy, V.L.N.; Ravikanth, V.; Yenkateswarlu, Y. Synth.Commun. 2003, 33, 1531-1536.
- Bandgar, B. P.; Makone, S.S.; Kulkarni, S.P. Monatsh. Chem.2000, 131, 417-420.
- Li, T. S.; Zhang, Z. H.; Gao, Y. J. Synth. Commun. 1998, 28, 4665-4671.
- (a) Kumar, P.; Hegde, V. R.; Kumar, T. P. Tetrahedron Lett. 1995, 36, 601-602. (b) Pereira, C.B.; Gigante, M. J.; Marcelo-Curto, H.; Carreyre, G.; Perot, G.; Guisnet, M. Synthesis1995, 1077-1078. (c) Ballini, R.; Bordoni, M.; Bosica, G.; Maggi, R.; Sartori, G. Tetrahedron Lett.1998, 39, 7587-7580.
- Olah, G. A.; Mehrotra, A. K.; Synthesis1982, 962-963.
- Jin, T. S.; Du, G. Y.; Li, T. S. Ind. J. Chem., Sec. B 1998, 939-940.
- Wang, C.; Li. M.; Synth. Commun. 2002, 32, 3469-3473.
- Gowravaram, S.; Abraham S.; Ramalingam, T.; Yadav, J. S. J. Chem. Res. (S)2002, 3, 144-146.
- Deka, N.; Borah, R.; Kalita, D. J.; Sarma, J. C. J. Chem. Res.(S)1998, 94-95.
- Agarwal, V. K.; Fonquerna, S. S.; Vennall, G. P. Synlett 1998, 849-850.
- Deka, N.; Kalita, D. J.; Borah, R.; Sarma, J. C. J. Org. Chem. 1997, 62, 1563-1564.
- Karimi, B.; Seradj, H.; Ebrahimian, R. G. Synlett2000, 623-624.
- Michie, J. K.; Miller, J. A. Synthesis1981, 824-824.
- Gregory, M. J. J. Chem. Soc. B 1970, 1201-1207.
- Chandra, K. L.; Saravanan, P.; Singh, V. K. Synlett 2000, 359-360.
- Sumida, N.; Nishioka, K.; Sato, T. Synlett2001, 12, 1921-1922.
- Jin, T. S.; Sun, G.; Li, Y. W.; Li, T. S. Green Chem. 2002, 4, 255-256.
- Yadav, J. S.; Reddy, B. V. S.; Srinivas, Ch. Synth.Commun.2002, 32, 2169-2174.
- Roy, S. C.; Banerjee, B. Synlett2002, 1677-1678.
- Karimi, B.; Maleki, J.; J. Org. Chem.2003, 68, 4951-4954.
- Ranu, B.C.; Dutta, J.; Das, A. Chem. Lett. 2003, 32, 366-367.
- Firouzabadi, H.; Iranpoor, N.; Nowrouzi, F.; Amani, K.; Tetrahedron Lett.2003, 44, 3951-3954.
- Smitha, G.; Reddy, Ch.S. Tetrahedron2003, 59, 9571-9576.
- Aggen, D.H.; Hayes, Arnold, J.N.; Hayes, P.D.; Smoter, N.J.; Mohan, R.S. Tetrahedron2004, 60, 3675-3679.
- Carrigan, M.C.; Eash, K.J.; Oswald, M.C.; Mohan, R.S. Tetrahedron Lett. 2001, 42, 8133-8135.
- Curini, M.; Epifano, F.; Marcotullio, M.C.; Rosati, O.; Nocchetti, M. Tetrahedron Lett. 2002, 43, 2709-2711.
- 37. Kumar, S.; Saini, A.; Sandhu, J. A. Arkivok2007, 14, 27-33.
- Sun, P.; Hu, Z. J. Chem. Res. 2005, 10, 659-660.
- Palacios-Grijalva, L. N.; Cruz-González, D. Y.; Lomas-Romero, L.; González-Zamora, E.; Ulibarri, G.; Negrón-Silva, G. E. Molecules2009, 14, 4065-4078.
- 41. Ghorbani-Vaghei, R.; Amiri, M.; Moshfeghifar, N.; Veisi, H.; AkbariDadamahaleh, S. J. Iran. Chem. Soc. 2009, 6, 754-760.
- Shirini, F.; Mamaghani, M.; Mostashari-Rad, T.; Abedini, M. Bull. Korean Chem. Soc.2010, 31, 2399-2401.
- Mirjalili, B. F.; Zolfigol, M. A.; Bamoniri, A.; Sheikhana, N. J. Chin. Chem. Soc. 2006, 53, 955-959.
- Zhang, X.; Li, L.; Zhang, G. Green Chemistry2003, 5, 646-468.
- Dalpozzo, R.; Nino, A. D.; Maiuolo, L.; Nardi, M.; Procopio, A.; Russo, B.; Tagarelli, A. Arkivoc2006, 6, 181-189.
- Shelke, k.; Sapkal, S.; Kategaonkar, A.; Shingate, B.; Shingare, M .S. S. Afr. J. Chem. 2009, 62, 109-112.
- Negrón, E. E.; Palacios, L. N.; Angeles, D.; Lomas, L.; Gaviño, R. J. Mex. Chem. Soc.2005, 49, 252-256.
- Heravi, M. M.; Bakhtiari, K.; Taheri, S.; Oskooie, H. A. Green Chem. 2005, 7, 867-869.
- Pourmosavi, S. A.; Zinati, Z. Turk. J. Chem. 2009, 33, 385-392.
- Yin, L.; Zhang, Z. H.; Wang, Y. M.; Pang, M. L. Synlett2004, 10, 1727-1730.
- Khan, A. T.; Choudhury, L. H.; S. Ghosh, J. Mol. Catal. A: Chem. 2006, 255, 230-235.
- Ziyaei, A.; Azizi, N.; Saidi, M. R. J. Mol. Catal. A: Chem. 2005, 238, 138-141.
- Kumar, R.; Thilagavathi, R.; Gulhane, R.; Chakraborti, A. K. J. Mol. Catal. A: Chem. 2006, 250, 226-231.
- Hajipour, A. A.; Khazdooz, L.; Ruoho, A. E. Catal. Commun.2008, 9, 89-96.
- Setamdideh, D.; Khezri, B.; Esmaeilzadeh, S. J. Chin. Chem. Soc. 2012, 59, 1119-1124.
- Setamdideh, D.; Khezri, B.; Alipouramjad, A. J. Chin. Chem. Soc. 2013, 60, 590-596.
- Sofighaderi, S.; Setamdideh, D. Orient. J. Chem. 2013, 29, 1135-1137.
- Setamdideh, D.; Sepehraddin, F. J. Mex. Chem. Soc. 2014, 58, 22-26.
- Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M.; Aliporamjad, A. Asian J. Chem.2012, 8, 3591-3596.
- Setamdideh, D.; Rahmatollahzadeh, M. J. Mex.Chem. Soc. 2012, 56, 169-175.
- Kamari, R.; Setamdideh, D. Orient. J. Chem.2013, 29, 497-499.
- AziziAsl, P.; Setamdideh, D.J. Chin. Chem. Soc. 2014, 61, 940-944.
- Setamdideh, D. J. Mex. Chem. Soc. 2014, 58, 230-234.

This work is licensed under a Creative Commons Attribution 4.0 International License.









