One-pot three-component synthesis of spirooxindoles catalyzed by nano Ag/kaolin
Bahareh Sadeghi1, Zahra Lasemi2 and Razieh Azimi3
1Department of Chemistry, Yazd Branch, Islamic Azad University, P.O. Box 89195-155, Yazd, Iran
2Department of Chemistry, Firoozkooh Branch, Islamic Azad University, Firoozkooh, Iran
3Department of Organic Chemistry, Faculty of Chemistry, University of Mazandaran, Babolsar, Iran
DOI : http://dx.doi.org/10.13005/ojc/310272
Article Received on :
Article Accepted on :
Article Published : 03 May 2015
Nano Ag/kaolin has been used as an effective heterogeneous catalyst for one-pot synthesis of spiro[4H-pyran-3,3'-oxindoles] via reaction of isatin derivatives, malononitrile and cyclic 1,3-diketones under mild reaction conditions in excellent yields.
KEYWORDS:Spirooxindoles; Heterogeneous catalyst; Cyclic 1;3-diketone; Nano Ag/kaolin; Malononitrile
Download this article as:| Copy the following to cite this article: Sadeghi B, Lasemi Z, Azimi R. One-pot three-component synthesis of spirooxindoles catalyzed by nano Ag/kaolin. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Sadeghi B, Lasemi Z, Azimi R. One-pot three-component synthesis of spirooxindoles catalyzed by nano Ag/kaolin. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8742 |
Introduction
Multicomponent reactions forming heterocyclic compounds are powerful tools in the drug discovery process as they can offer expedient synthesis of libraries of drug like compounds in single operation1.
Spirooxindoles occupy an important place in the area of heterocyclic chemistry because they are frequently found in numerous natural and synthetic products along with useful biopharmaceutical, physiopharmaceutical, and pharmaceutical activities2-7.
In recent years, several improved protocols for the synthesis of spirooxindoles with fused chromenes have been reported by modification of three-component condensation of isatin derivatives, activated methylene reagents, and 1,3-dicarbonyl compounds using various catalysts such as triethylbenzyl ammonium chloride (TEBA)8, InCl39, NEt310, electrogenerated base (NaBr/ROH)11, β-cyclodextrin12, [BMIm]BF413, L-proline14 ,MgO15, surfactant metal carboxylates16, ethylenediaminediacetate17, Carbon–SO3H18, ZnS NPs19. Although all of these methods are effective, but some of them have drawbacks such as, harsh reaction conditions9, long reaction times8,12, technical intricacy11, use of expensive13, unsafe10, and unreusablecatalysts8,10,16,17. Therefore, the development of a new and simple synthetic method for the preparation of spirooxindole derivatives has become an interesting challenge.
During the recent years, the use of heterogeneous catalysts has received considerable importance in organic synthesis because of their environmental, economical and industrial aspects20. Metal colloids, mineral clays and supported reagents on silica gel, alumina and other solid supports are some common examples of heterogeneous catalysts that have extensive applications in organic transformations. These catalysts have attracted a great deal of attention due to their ease of handling, enhanced reaction rates, greater selectivity and simple work up in most cases21.
As a part of our ongoing efforts towards the development of new procedure for the synthesis of spirooxindoles through multi component reaction, we have discovered an effcient and environmentally friendly procedure for the synthesis of spiro[4H-pyran-3,3′-oxindole] derivatives. We report herein, for the first time, a simple, mild and expeditious synthesis of spirooxindoles in high yields using nano Ag/kaolin as a catalyst in ethanol (Scheme 1).
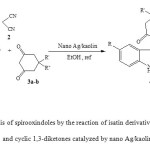 |
Scheme1: Synthesis of spirooxindoles by the reaction of isatin derivatives, with malononitrile and cyclic 1,3-diketones catalyzed by nano Ag/kaolin. Click here to View scheme |
Experimental
The chemical materials used in this work were obtained from Merck and Aldrich and used without purification. 1H NMR spectra was recorded on a Bruker DRX-400 AVANCE spectrometer at 400 MHz. The powder X-ray diffraction studies were made on Philips PW3719 X-ray diffractometer by using Cu-Kα radiation. Transmission electron microscopy (TEM) images were obtained using a Phlips CM10 microscope operated at 200 kV. Melting points were determined on a thermo scientific IA 9200 and are uncorrected.
General Procedure for the Synthesis of Spirochromenes (4)
A mixture of substituted isatins (1 mmol), cyclic 1,3-diketone (1 mmol), malononitrile (1 mmol) and nano Ag/kaolin (0.085 g, 7 mol%) in EtOH (10mL) was stirred at reflux temperature. Upon compilation, monitored by TLC (n-hexane/ethyl acetate: 2/1), the reaction mixture was allowed to cool to room temperature. The catalyst was separated by filtration of this solution. The solution was concentrated under vacuum to afford the product, which was purified by recrystallization in the ethanol. All the products were identified by comparing of melting point and 1H NMR spectra with those of authentic samples reported in the literature.
2-Amino-5′-Nitro-2′,5-Ioxo-5,6,7,8-Tetrahydrospiro[Chrom-ene-4,3′-Indoline]-3-Carbonitrile (4c)
m.p. 283-285 ºC; 1H NMR (400 MHz, DMSO-d6) δ: 1.79 -2.02 (m, 2H, CH2), 2.22-2.26 (m, 2H, CH2), 2.62-2.69 (m, 2H, CH2), 7.03 (d, J=8.6 Hz, 1H), 7.40 (brs, 2H, NH2), 7.87 (s, 1H, ArH), 8.13 (d, J= 6.22 Hz, 1H, ArH), 11.17 (s, 1H, NH).
Amino-5′-chloro-2′,5-dioxo-5,6,7,8-tetrahydro-spiro[chromene-4,3′-indoline]-3-carbonitrile (4d)
m.p. 293–294 ºC (dec); 1H NMR (400 MHz, DMSO-d6) δ: 1.89-2.00 (m, 2H, CH2), 2.21 (t, J = 6.8 Hz, 2H, CH2), 2.64 (t, J = 6.4 Hz, 2H, CH2), 6.81 (d, J = 8.4 Hz, 1H, ArH), 7.18 (d, J = 2.0 Hz, 1H, ArH), 7.24 (d, J = 8.0 Hz, 1H, ArH), 7.31(brs, 2H, NH2), 10.57 (s, 1H, NH).
2-Amino-1′,2′,5,6,7,8-hexahydro-5′,7,7-trimethyl-2′,5-dioxospiro[4H-1-benzopyran-4,3′-[3H]indole]-3-carbonitrile (4f)
m.p. 275-277 ºC; 1H NMR (400 MHz, DMSO-d6) δ: 0.99 (s, CH3 ), 1.03 (s, CH3), 2.11-2.15 (m, CH2), 2.19 (s, CH3), 2.50-2.53 (m, CH2), 6.68 (d, J=7.6, 1H, ArH), 6.75 (s, 1H, ArH), 6.90 (d, J=10.4, 1H, ArH), 7.14 (br. s, NH2), 10.26 (s, NH).
2-Amino-5′-Chloro-8,8-Dimethyl-2′,5-Dioxo-5,6,7,8-Tetrahydrospiro[Chromene-4,3′-Indoline]-3-Carbonitrile (4h)
m.p. 288-289 ºC; 1HNMR (400 MHz, DMSO-d6) δ: 1.01 (s, 6H, CH3), 2.11(s, 2H, CH2), 2.60-2.43 (m, 2H, CH2), 6.79 (d, J=8.1Hz, 1H, ArH), 7.06 (s, 1H, ArH), 7.16 (d, J=8.2 Hz, 1H, ArH), 7.27 (brs, 2H, NH2), 10.65 (s, 1H, NH).
Results and Discussion
Initially, nano Ag/kaolin was prepared by the method described in literature.22 In continution of our investigations of the application of solid acids in organic synthesis, 23-25 we bought a mild and convenient method for the synthesis of spiro[4H-pyran-3,3′-oxindoles]. Our investigation began with the evaluation of nano Ag/kaolin as a catalyst in the reaction of 5-bromoisatin (1 mmol), malononitrile (1 mmol), and 1,3-cyclohexadione (1 mmol) in EtOH. The use of 20 mol% of nano Ag/kaolin in this condition afforded a 94% yield (Table 1, entry 5) of the desired product.
 |
Table1: Optimization of reaction conditions for the synthesis of 4a Click here to View table |
At the same time a decline in yield was observed by using kaolin as catalyst (Table 1, entry 9). In the absence of catalyst, the reaction proceeded sluggishly (Table 1, entry 10). The influence of other solvents was also examined. The above experiment was performed in various solvents including tetrahydrofuran, acetonitrile, water, dichloromethane, and ethanol (Table 1, entries 1-5). Of these solvents, ethanol appears to give the best results. The effects of temperature were also examined on model reaction. Decreasing of temperature to 25 ºC afforded the product 4a in low yields (Table 1, entry 8). The dimensions of nano Ag/kaolin were observed with TEM (Figure 1).
 |
Figure1: TEM image of nano Ag/kaolin Click here to View figure |
To study the scope of the reaction, a series of substituted isatins, different cyclic 1,3-diketones and malononitrile were reacted using nano Ag/kaolin in refluxing EtOH (Table 2). From the results, it is evident that all of the reactions provided the desired spirooxindole products in good to excellent yields employing both electron-deficient (Table 2, entries 1, 3-5, 7,8) and electron-rich (Table 2, entries 2,6) isatins as substrates. Though 1,3-cyclohexadione and 5,5-di methyl-1,3-cyclohexadione gave good results, 5,5-di methyl-1,3-cyclohexadione gave lower yields and longer reaction time.
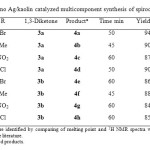 |
Table2: Nano Ag/kaolin catalyzed multicomponent synthesis of spirooxindoles (4) |
Finally, the recovery and reusability of the catalyst was investigated in model reaction. After completion of reaction the mixture was filtered off to separate the catalyst and then dry the solid residue. As can be seen from Table 1, the catalyst was recovered and reused for at least two consecutive runs without significant loss of activity (Table 1, entries 6-7).
In summary, a mild and efficient method for the synthesis of spiro-heterocyclic compounds via one-pot three-component reaction catalyzed by nano Ag/kaolin is reported. The salient features of this procedure are high yield, short reaction times, operational simplicity, and easy separation.
References
- Hulme C.; Gore, V. Curr. Med. Chem. 2003, 10, 51-80.
- Galliford C. V.; Scheidt, K. A. Angew. Chem. Int. Ed. 2007, 46, 8748-8758.
- Abdel-Rahman, A. H.; Keshk, E. M.; Hanna M. A.; El-Bady, S. M. Bioorg. Med. Chem. 2004, 12, 2483-2488.
- Dandia, A.; Singh, R.; Khaturia, S.; Merienne, C.; Morgant G.; Loupy, A. Bioorg. Med. Chem. 2006, 14, 2409-2417.
- Badillo, J. J.; Hanhan N. V.; Franz, A. K. Curr. Opin. Drug. Discovery. Dev. 2010, 13, 758-776.
- Ding, K.; Lu, Y. P.; Nikolovska-Coleska, Z.; Wang, G. P.; Qiu, S.; Shangary, S.; Gao, W.; QinStuckey, D. G. J.; Krajewski, K.; Roller P. P.; Wang, S. M. J. Med. Chem. 2006, 49, 3432-3435.
- Antonchick, A. P.; Gerding-Reimers, C.; Catarinella, M.; Schurmann, M.; Preut, H.; Ziegler, S.; Rauh, D.; Waldmann, H. Nat. Chem. 2010, 2, 735-740.
- Zhu, S. L.; Ji, S. I.; Zhang, Y. A. Tetrahedron 2007, 63, 9365-9372.
- Shanthi, G.; Subbulakshmi G.; Perumal, P. T. Tetrahedron 2007, 63, 2057-2063.
- Litvinov, Y. M.; Mortikov V. Y.; Shestopalov, A. M. J. Comb. Chem., 2008, 10, 741-745.
- Elinson, M. N.; Ilovaisky, A. I.; Merkulova, V. M.; Demchuk, D. V.; Belyakov, P. A.; Ogibin Y. N.; Nikishin, G. I. Electrochimica. Acta. 2008, 53, 8346-8350.
- Sridhar, R.; Srinivas, B.; Madhav, B.; Reddy, V. P.; Nageswar Y. V. D.; Rao, K. R. Can. J. Chem. 2009, 87, 1704-1707.
- Rad-Moghadam, K.; Youseftabar-Miri, L. Tetrahedron 2011, 67, 5693-5699.
- (a) Yuling, L.; Hui, C.; Chunling, S.; Daqing, S.; Shunjun, J. J. Comb. Chem. 2010, 12, 231-237; (b) Raghuvanshi, D. S.; Singh, K. N. J. Heterocycl. Chem. 2010, 47, 1323-1327.
- Karmakar, B.; Nayak, A.; Banerji, J. Tetrahedron Lett. 2012, 53, 5004-5007.
- Wang, L. M.; Jiao, N.; Qiu, J.; Yu, J. J.; Liu, J. Q.; Guo F. L.; Liu, Y. Tetrahedron 2010, 66, 339-343.
- Lee, Y. R.; Hari, G. S. Synthesis 2010, 453-464.
- Maheshwar Rao, B.; Niranjan Reddy, G.; Vijaikumar Reddy, T.; Prabhavathi Devi, B. L. A.; Prasad, R. B. N.; Yadav, J. S.; Subba Reddy, B. V. Tetrahedron Lett. 2013, 54, 2466-2471.
- Dandia, A.; Parewa, V.; Kumar Jain, A.; Rathore, K. S. Green Chem. 2011, 13, 2135-2145.
- Tamagaki, S.; Card, R. J.; Neckers, D. C. J. Am. Chem. Soc. 1978, 100, 6635-6639.
- Mohammadpoor-Baltork, I.; Mirkhani, V.; Moghadam, M.; Tangestaninejad, Sh.; Zolfigol, M. A.; Abdollahi-Alibeik, M.; Khosropour, A. R.; Kargar, H.; Hojati, S. F. Catal. Commun. 2008, 9, 894-901.
- Hashemian, S.; Shahedi, M. R. J. Chem. 2013, 2013, Article ID 285671, 7 pages, http://dx.doi.org/10.1155/2013/285671.
- Sadeghi, B.; Namakkoubi, A. Hassanabadi, A. J. Chem. Res. 2013, 37, 11-13.
- Sadeghi, B. J. Chem. Res. 2013, 37, 171-173.
- Sadeghi, B.; Farokhi Nezhad, P.; Hashemian, S. J. Chem. Res. 2014, 38, 54-62.
- Wang, G. D.; Zhang X. N.; Zhang, Z. H. J. Heterocycl. Chem. 2013, 50, 61-65.

This work is licensed under a Creative Commons Attribution 4.0 International License.









