NMR Shielding Tensors and Thermodynamic Investigation of B28N28 Nano-cone Structure: A molecule for Fe3+Capturing
Hadi Lari*1, Ebrahim Balali2
1Department of chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran
2Young Researchers & Elite club, pharmaceutical Sciences Branch, Islamic Azad University Tehran, Iran
Corresponding author:
Email: hadilari1359@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/310207
Article Received on :
Article Accepted on :
Article Published : 19 Jun 2015
M06&B3LYP/3-21G/6-31G/6-31G*/6-311G* density functional theory (DFT) and HF/3-21G/6-31G/6-31G*/6-311G *ab-initio calculations have performed for the structure and stability of B28N28 nano-cone. In this work, it was calculated the geometrical structure, and stability to predict NMR and thermodynamics parameters. We have found these kinds of nano-cone are useful for capturing of Fe3+ Ion
KEYWORDS:Fe3+ Ion; Density functional theory (DFT); Ab-initio calculation; Geometrical structure; Thermodynamic parameters; Active sites
Download this article as:| Copy the following to cite this article: Lari H, Balali E. NMR Shielding Tensors and Thermodynamic Investigation of B28N28 Nano-cone Structure: A molecule for Fe3+Capturing. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Lari H, Balali E. NMR Shielding Tensors and Thermodynamic Investigation of B28N28 Nano-cone Structure: A molecule for Fe3+Capturing. Available from: http://www.orientjchem.org/?p=9309 |
Introduction
DFT (density functional theory) is one of the computational methods which can be used in different systems and it is more useful for some calculations than other methods. It is clear that basis sets are vast various. Primarily discovery of C60 has led to synthesizing higher fullerenes, carbon nanotubes, and other non-carbon nanostructures such as BN nanotubes .Although BN neocons have been known since 1994, we have been able to observe these structures experimentally until recently [1-6].
The carbon nanotube (CNT) is a representative nano-material. CNT is a cylindrically shaped carbon material with a nano-metric-level diameter [10-20].
Its structure, which is in the form of a hexagonal mesh, resembles a graphite sheet and it carries a carbon atom located on the vertex of each mesh. The sheet has rolled and its two edges have connected seamlessly [6-15].
Although it is a commonplace material using in pencil leads, its unique structure causes it to present characteristics that had not found with any other materials. CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite [16-20].
The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers. The length is usually in the order of microns, but single-wall CNT with a length in the order of centimeters has recently released [19-25].
CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite. The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers [20-26].
The length is usually in the order of microns, but single-wall CNT with a length about centimeters have recently released. The extremities of the CNT have usually closed with lids of the graphite sheet [21-30].
The lids consist of hexagonal crystalline structures (six-membered ring structures) and a total of six pentagonal structures (five-membered ring structures) placed here and there in the hexagonal structure [22-35]. The first report by Iijima was on the multi wall form, coaxial carbon cylinders with a few tens of nanometers in outer diameter [25-40]. Two years later single walled nanotubes were reported [8-15]. SWCNTs have considered as the leading candidate for nano-device applications because of their one-dimensional electronic bond structure, molecular size, and biocompatibility, controllable property of conducting electrical current and reversible response to biological reagents hence SWCNTs make possible bonding to polymers and biological systems such as DNA and carbohydrates [30-50].
boron nitride nanotube (BNNTs) have attracted much interests due to their large gap semi conducting character[41-55].Boron nitride (BN) is a structural existing in cubic (diamond-like), hexagonal (graphite-like), turbo static, and amorphous forms .these compounds have been produced by a variety of methods, such as arc melting[50-59], high temperature chemical reaction[44-60], carbon nanotube templates[50-65], and laser ablating[52-64] The most attention has been focused on the development of new methods for the production of nanotube and inorganic fullerene of other materials.
In addition, theoretical calculations have been described the possible existence of small BN clusters. Jensen and Toftlund [58-70] performed ab initio calculations for B28N28,c sters in different geometries. Based on density functional calculations it has also been proposed that other nanotube could be synthesized. [60-75]
Theoretical studies have been performed for fullerene-like B28 N28 clustersin which it has been found that a structure built from squares and hexagons is more stable than those built from pentagons and hexagons. This is because in the second case less stable B-B and N-N bonds are formed, [65-90].
The most stable B28N28 structure is built from six squares and eight hexagons. [70-102]
 |
Table1: HOMO and LUMO and Gap energy of 3 N28B28 Nanocone Click here to View table |
In this work, we focused on B28N28 nano-con. Our aim was to obtain the global minimum energy structure. For this structure, we use the hybrid B3LYP exchange-correlation functional within density functional theory. Primary, structure optimization calculated and then Nuclear Magnetic Resonance (NMR) parameters by density Functional Theory (DFT) method calculated on the optimized structure. Isotropic chemical shielding, anisotropic chemical shielding parameters at all of the atoms nuclei are presented in Table 1. And also, Thermodynamic Properties have been considered in Table 2
We have found that these kinds of Nano-cones are useful for Fe3+Capturing. In material sciences Boron nitride, which appears in a manifold of crystalline modifications, has been an extremely practical material with hexagonal and cubic boron nitride as most outstanding materials. The BN cluster is a polar molecule and BN nanotubes have an inert chemical structure. We can see that there is a negative charge at nitrogen atom and a positive charge at boron atom, so we can use an electrophilic or nucleophilic reagent as a solution for BN clusters.
BN nanotubes are very suitable for composite materials because these structures have a higher temperature resistance to oxidation than the carbon nanotubes. All the BN nanotubes are semiconductors. The BN nanotubes have the band gaps which can be greater than 2 eV for most tubes also we know that the smallest carbon nanotubes are semiconductor and these structures obtain the properties of graphite when the diameter of these structures increases but BN nanotubes are semiconductors without attention to the diameter. On the basis of the similarities in characteristics between carbon and BN-based (BN=boron nitride) substances, BN-based nanotubes can be stable and therefore their electronic structure can be studied. The comparison between BN nanotubes and carbon nanotubes shows that BN nanotubes have more interesting characteristics than the carbon nanotubes [60-100].
Recently the boron nitride (BN) nanoscale cone particles have been discovered and these structures are made up of conical shells without any seamless. Most of the studies about nanocones have been done so far with carbon structures. High-resolution transmission electron microscopy and nanobeam electron diffraction made the orientation of the BN hexagonal rings possible. Recently theoretical investigations on (BN) n nanocones have gained more attention in carbon nanotubes when there is not any experimental result [55-100].
Considering the above mentioned, BN nanotubes are very important and interesting for new research and can open a huge spectrum in the field of theoretical and experimental research. In the fig.1 structure of B28N28 is shown and this particular nanocone configuration has been proposed in this research.
Computational Method
The Gaussian 98 program was run to obtain the best prediction of this particular structure. Also all Ab-Initio and DFT (density functional theory) calculations were done with the Gaussian 98 program. Frequency analyses were carried out to show that the optimized structures are true minima or transition states on the potential energy surfaces of a specific structure without imaginary frequencies.
In this work, geometry optimizations in the gas phase for B28N28 were performed at density functional theory (DFT) level with B3LYP and Ab-Initio with HF (hartree fock) methods in different basis sets at the temperature of 298.15 K, The parameters were calculated for B28N28 in the gas phase in different methods and basis sets include thermodynamic and NMR parameters. The chemical shielding shows the phenomenon which is dependent on the secondary magnetic field which is built by the induced movements of the electrons which encompass the nuclei. The chemical shielding is built by a three-by-three matrix which is biodegraded into a single scalar term, three antisymmetric pseudo vector components, and five components which correspond to a symmetric tensor. It can be observed the single scalar and the five symmetric tensor elements in the normal NMR spectra of the solids.
The chemical shielding tensor includes the chemical shift isotropy (CSI) and chemical shift anisotropy (CSA) and the anisotropy (Δб) of the tensor, the shielding tensor asymmetry parameter (η) and chemical shift (δ) are calculated.
The thermodynamic parameters that were calculated in this research are Gibbs free energy, enthalpy, internal energy (It is clear that the sum of zero point energy (ZPE) and thermal energy is internal energy.) and entropy then these reports were compared with each other in order to obtain the best results. These results were reported in tables.
Results and Discussion
The results are listed in tables 1-3, and the figures are explained in figs 1-4. The geometry optimization for B28N28 nano-cone has been done with HF and B3LYP methods at different basis sets such as 4-31 G, 6-31 G, 6-31 G* and 6-311 G*. Then thermodynamic properties were calculated for this structure in gas phase at 298.15K in the same methods and basis sets. A comparison of Gibbs free energy (G), Enthalpy (H), Entropy (S) and Internal energy (E) in different methods and basis sets are shown in table 2. As shown in table 2, the maximum values for Gibbs free-energy (G) , Enthalpy (H) and Internal energy (E) were calculated when 6-311G* basis set had been applied at B3LYP method.
Table2: Thermodynamic properties in different methods and basis sets, for B28N28
without and including Fe3+ at 298.15K in gas phase)
|
H(kcal/mol)– Relative |
-G(kcal/mol) Relative |
Relative E(kcal/mol) – |
Basis set |
Methods |
|
|
0.0 |
0.0 |
0.0 |
4-31g
|
HF
|
|
|
1.99 |
2.33 |
2.25 |
6-31g
|
||
|
2.05 |
3.55 |
3.32 |
6-31g*
|
||
|
3.22 |
3.96 |
3.45 |
6-311g*
|
||
|
0.0 |
0.0 |
0.0 |
4-31g
|
B3LYP
Including Fe3+ |
|
|
1089 |
2.55 |
3.33 |
6-31g
|
||
|
2.77 3.43 5.34 |
3.13 4.01 6.22 |
3.62 3.99 5.66 |
6-31g*
6-311g*
6-31g* |
||
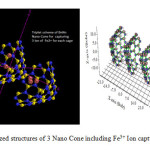 |
Figure1: the optimized structures of 3 Nano Cone including Fe3+ Ion capturing. Click here to View figure |
 |
Figure2: Shaded Surface map with projection for electron density of 3 nano-cone of B28N28 Click here to View figure |
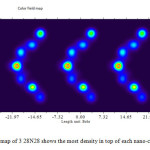 |
Figure3: Color map of 3 28N28 shows the most density in top of each nano-cone Click here to View figure |
 |
Figure4: The line of contour map Click here to View figure |
According to the results that are shown in table2, the largest values have been obtained in B3LYP method.
Considering the optimized structure, the NMR shielding tensors were calculated then these parameters were used to show active sites in this structure. The results of бiso, бaniso, δ, Δб and η for this nanocone in the same methods and basis sets are shown in table 3.Finally the charts of бiso, бaniso, δ and η for the atoms of B10N11 in the 4-31G, 6-31G, 6-31G*, 6-311G* level of theory and B3LYP and HF methods.We can obtain the interesting results from the NMR charts. Comparison of these charts (бiso, бaniso, δ and η) shows that some of peaks in these charts are similar to each other. If these peaks are reviewed, we can understand which similar atoms are situated in the same peaks of different charts. The comparison of these peaks shows that three atoms are exactly repeated in бiso, бaniso, δ and η charts. These three atoms are the active sites in this structure in B28N28
Table3: NMR parameters: including for B28N28in different methods and basis sets
|
HF |
Methods |
|||||||||||
|
B3LYP |
||||||||||||
|
168N |
150N |
28B |
17N |
Fe3+ |
20B |
10B |
102N |
1B |
101N |
100N |
Atoms |
|
|
104.68 |
158.4 |
129.73 |
178.59 |
178.58 |
96.68 |
101.1 |
183.8 |
105.7 |
158.5 |
104.6 |
σiso |
4-31g |
|
105.2 |
96.3 |
91.3 |
157.46 |
157.44 |
88. 6 |
95. 5 |
154. 8 |
98.3 |
96.7 |
110.2 |
||
|
168.0 |
29.9 |
115.7 |
84.78 |
84. 1 |
82.0 |
129. 3 |
158. 0 |
52.5 |
29.63 |
168.0 |
σaniso |
|
|
164.09 |
60.6 |
115.7 |
103.54 |
103.58 |
66. 0 |
114. 5 |
159. 9 |
47.1 |
60.9 |
164.9 |
||
|
168.05 |
-41.9 |
115.73 |
84.75 |
84.75 |
82.98 |
129.6 |
158.9 |
-70.5 |
-41.7 |
168.0 |
Δσ |
|
|
164. 1 |
-61.4 |
113.7 |
103.5 |
103.59 |
66. 3 |
114. 6 |
159.89 |
-62. 5 |
-61.7 |
164.6 |
||
|
0.44 |
0.41 |
0.45 |
0.54 |
0.54 |
0.51 |
0.153 |
0.26 |
0.57 |
0.46 |
0.44 |
η |
|
|
0.91 |
0.96 |
0.616 |
0.162 |
0.166 |
0.87 |
0.32 |
0.24 |
0.53 |
0.99 |
0.94 |
||
|
112.03 |
-27.9 |
77.1 |
56.55 |
56.54 |
55.3 |
86.4 |
105. 3 |
-46. 8 |
-27. 8 |
1125 |
δ |
|
|
109.9 |
-40. 6 |
75.4 |
69.0 |
69.06 |
44.47 |
76.26 |
106. 6 |
-41. 9 |
-40.5 |
109.4 |
||
|
92.04 |
145. 6 |
78. 9 |
145. 4 |
104.53 |
92.0 |
80.4 |
168. 4 |
88.2 |
165. 9 |
165.6 |
σiso |
6-31g |
|
-20.68 |
78. 3 |
77. 2 |
78.31 |
64.24 |
-20.7 |
83.3 |
136. 5 |
88. 5 |
139.1 |
139.8 |
||
|
173.1 |
46. 8 |
99. 6 |
46.9 |
130.1 |
173.9 |
155.5 |
176. 4 |
60.2 |
96.3 |
96.3 |
σaniso |
|
|
179.06 |
59.48 |
76. 0 |
59.6 |
135.5 |
179.06 |
126.7 |
179. 2 |
54.52 |
113.5 |
113.2 |
||
|
173. 1 |
-52.05 |
99.4 |
-52.6 |
130.1 |
173.9 |
155.59 |
176.8 |
-79.9 |
96.3 |
96.3 |
Δσ |
|
|
179. 6 |
-59.5 |
76.5 |
-59.5 |
135.5 |
179.03 |
126.71 |
179.61 |
-70.3 |
113. 4 |
113.2 |
||
|
0.51 |
0.783 |
0.57 |
0.76 |
0.368 |
0.51 |
0.19 |
0.26 |
0.50 |
0.44 |
0.45 |
η |
|
|
0.799 |
0.99 |
0.8 |
0.96 |
0.53 |
0.793 |
0.33 |
0.20 |
0.54 |
0.16 |
0.15 |
||
|
115.9 |
-35. 7 |
66.37 |
-35. 9 |
86. 1 |
115.9 |
103.6 |
117.27 |
-53.3 |
64.2 |
64.2 |
δ |
|
|
119.3 |
-39. 7 |
50. 7 |
-39.6 |
90. 9 |
119.3 |
84.5 |
119.7 |
-47. 8 |
75. 1 |
75.4 |
||
|
94.05 |
165.26 |
155.21 |
183.4 |
126. 7 |
85. 5 |
88. 7 |
172. 7 |
172. 9 |
164.19 |
102. 4 |
σiso |
6-31g* |
|
84. 9 |
109.57 |
129.1 |
152.03 |
86. 0 |
75.55 |
83.3 |
148.12 |
148. 4 |
109.56 |
30.63 |
||
|
56.60 |
45.18 |
97.3 |
170. 9 |
127.7 |
95. 1 |
155. |
93.06 |
93.02 |
45.19 |
174.74 |
σaniso |
|
|
54.29 |
46. 6 |
114.6 |
171.0 |
125.5 |
85.0 |
141.8 |
106. 2 |
106. 1 |
46. 4 |
172.19 |
||
|
-76.2 |
-62. 7 |
97.3 |
170.8 |
127.7 |
95. 2 |
155. 5 |
93.0 |
93.09 |
-62.45 |
174.7 |
Δσ |
|
|
-71. 3 |
46. 6 |
114. 7 |
171.0 |
125.5 |
85.04 |
141. 5 |
106.6 |
106. 9 |
46.2 |
172.16 |
||
|
0.48 |
0.445 |
0.47 |
0.35 |
0.43 |
0.56 |
0.147 |
0.45 |
0.41 |
0.44 |
0.526 |
η |
|
|
0.57 |
0.1868 |
0.21 |
0.3 |
0.48 |
0.69 |
0.25 |
0.242 |
0.28 |
0.186 |
0.452 |
||
|
-50. 67 |
-41. 8 |
64. 50 |
113. 7 |
85. 3 |
63. 5 |
103.4 |
62.0 |
62. 7 |
-41. 9 |
116. 6 |
δ |
|
|
-47. 5 |
30. 1 |
76. 1 |
114.0 |
83. 7 |
56.6 |
94.3 |
71.1 |
71. 7 |
30. 3 |
114.7 |
||
|
147.89 |
147.8 |
82. 6 |
155.2 |
106.2 |
76.93 |
79.5 |
167.4 |
86.9 |
102.3 |
82.31 |
σiso |
6-311g* |
|
89.3 |
89.0 |
4.3 |
129.19 |
61.6 |
63.6 |
71.1 |
132.0 |
74.9 |
30.4 |
4.3 |
||
|
49.8 |
49.8 |
186.6 |
97.35 |
129.4 |
103.7 |
167.5 |
178.13 |
62.9 |
174.6 |
186.1 |
σaniso |
|
|
50.11 |
50.14 |
186.97 |
114.4 |
132.4 |
94.83 |
156.25 |
184.6 |
60.9 |
172.0 |
186. 4 |
||
|
-67.56 |
-67.5 |
186.6 |
97.3 |
129.7 |
103.3 |
167.5 |
178.3 |
-81.6 |
174.7 |
186.6 |
Δσ |
|
|
50.2 |
50.1 |
186.9 |
114. 5 |
132.26 |
94.8 |
156.2 |
184.2 |
-78.9 |
172.1 |
186.9 |
||
|
0.47 |
0.47 |
0.51 |
0.49 |
0.46 |
0.52 |
0.14 |
0.36 |
0.59 |
0.5 |
0.5 |
η |
|
|
0.13 |
0.13 |
0.41 |
0.22 |
0.49 |
0.68 |
0.21 |
0.3 |
0.54 |
0.45 |
0.48 |
||
|
-45.04 |
-45.0 |
124. 9 |
64.90 |
86. 2 |
68. 4 |
111.7 |
118. 5 |
-54. 0 |
116.4 |
124.4 |
δ |
|
|
42.4 |
33.43 |
124.6 |
76. 76 |
88.19 |
63.27 |
104.16 |
123.08 |
-52.6 |
114.76 |
124.64 |
||
In general, the chart of electronic charge in different methods and basis sets is similar to the charts of NMR parameters Nitrogen atoms have more electrons than Boron atoms therefore the location of negative electronic charge is on Nitrogen atoms and positive electronic charge is situated on Boron atoms. It is clear that Nitrogen atoms will be active sites in this structure.
 |
Figure5: Relief map shows the situation of 3 nano-cones versus distance Click here to View figure |
Conclusion
In summary, the stability of B28N28 was investigated. It is found that the amount of Gibbs free energy (G) , Enthalpy (H) and internal Energy (E) obtained in B3LYP/6-311G* level in the gas phase ( 298.15K ) are the largest amount and also optimization of B28N28 nano-cone at the B3LYP/6-311G*is suitable for this structure. The NMR data and the thermodynamics results indicate that this kind of nano-cone is suitable for capturing Fe3+ ion.
References
- Kroto, H. W.; Health, J. R.; O’Brien, S. C.; Curl, R. F.; Smalley, R. E. C60: Buckminsterfullerene. Nature (London), 1985, 318, 162
- Iijima Sumio. Nature (London), 1991. 354, 56.
- Chopra, Nasreen G.; Luyken , R. J.; Cherrey , K.; Crespi , Vincent H.; Cohen, Marvin L.; Louie, Steven G.; Zettl , A. Science,1995, 269, 966.
- Rubio Angel; Corkill , Jennifer L.; Cohen , Marvin L. Phys. Rev. 1994. B, 49, 5081
- Bourgeois,L.; Bando,Y.; Han, W.Q.; Sato, T.Phys. Rev. 2000, B, 61, 7686.
- Terauchi, M.; Tanaka, K.; Suzuki, A.; Ogino, K.; Kimura, Chem. Phys. Lett. 2000, 324, 359.
- Sachdeva, H, Frank Müllera, F .; Stefan Hüfnerb, S. Diamond and Related Materials, 2010. 19, 1027-1033.
- Massimo, Fusaro .:Quantum Matter,2014, 3, 481-487
- Micheal Arockiaraj , Rev. Theor. Sci. 2014, 2, 261-273
- Martin Bohlén .; Kim Bolton ,Quantum Matter, 2014, 3, 339-343
- Monajjemi, M.; Baei, M.T.; Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9), 1430-1437
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Seyed Hosseini, M. Journal of Computational and Theoretical Nanoscience .2013 ,10 (10), 2473-2477
- Yahyaei ,H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346–361
- Monajjemi, M .; Jafari Azan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures.2013, 21(6), 503–515
- Bhupesh, Bishnoi .; Bahniman ,Ghosh .Quantum Matter.2014, 3, 469-475
- Sule, Celasun , Rev. Theor. Sci.2013, 1, 319-343
- Akshaykumar, Salimath .; Bahniman, Ghosh, Quantum Matter, 2014, 3, 72-77
- Nafisi, S.; Monajemi, M.; Ebrahimi, S. Journal of Molecular Structure. 2004,705 (1-3) 35-39
- Monajjemi , M.; Baheri ,H.; Mollaamin ,F. Journal of Structural Chemistry.2011 52(1), 54-59
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
- Medhat Ibrahim and Hanan Elhaes ,Rev. Theor. Sci. 2013, 1, 368-376
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22 ( 4 ), 673-692
- Monajjemi, M. Struct. Chem, 2012, 23 551.
- Monajjemi , M.; Lee, V.S. ; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F, J. Phys.Chem. C. 2010, 114 (2010) 15315
- Davide Fiscaletti and Amrit Sorli ,Quantum Matter,2014 3, 200-214
- Monajjemi , M.; Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. Afr. J. Pharm. Pharmacol .2010, 4 (8), 521-529
- Bjürn Piglosiewicz, Jan Vogelsang.;Slawa Schmidt.; Doo Jae Park.; Petra Groß, .; Christoph Lienau ,Quantum Matter,2014 ,3, 297-306
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Fazaeli ,R.; Monajjemi ,M.; Ataherian ,F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Mollaamin, F, J Clust Sci, 22(2011)673.
- Anurag Srivastava.; Nileshi Saraf.; A. K. Nagawat , Quantum Matter,2013, 2, 401-407
- IIJIMA Sumio.; YUDASAKA Masako.; NIHEY Fumiyuki.; NEC TECHNICAL JOURNAL,2007, 2,1,
- S. Iijima and T. Ichihasi, Nature,1993,363, 603
- D. S. Bethune.;C. H. Kiang.; M. S. deVries.; G. Gorman, R. Savoy.; J. Vazques.; R. Beyers, Nature ,1993, 363, 605
- Monajjemi, M .; Aghaie , H.; Naderi , F. Biochemistry (Moscow).2007, 72 (6), 652-657
- Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
- Davide Fiscaletti,Rev. Theor. Sci.2013 1, 103-144
- Monajjemi , M.; Chahkandi ,B.; Zare,K.; Amiri, A. Biochemistry (Moscow),2005 70 (3), 366-376
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007, 2,1956-1963
- Mollaamin , F.; Monajjemi , M , Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci , 2011,6, 1496-1500
- Monajjemi, M .; Faham, R.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures , 2012 20, 163–169
- Monajjemi, M.; Khaleghian, M.; Tadayonpour, N.; Mollaamin, F. International Journal of Nanoscience, 2010, 9 (05), 517-529
- Mollaamin , F .; Najafi ,F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi ,M. Fullerenes, Nanotubes, and Carbon Nanostructures,2011 19, 653–667
- Monajjemi, M.; Chegini , H. ; Mollaamin , F. ; Farahani ,P, Fullerenes, Nanotubes, and Carbon Nanostructures.2011,19, 469–482
- Monajjemi, M.; Yamola ,H.; Mollaamin,F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Monajjemi, M.; Heshmata, M.; Haeri, HH , Biochemistry (Moscow),2006, 71 (1), S113-S122
- Xinjun Wang.; Yi Xie and Qixun Guo , CHEM. COMMUN. 2003, 2688–2689
- Rubio.; J.L. Corkill.; M.L. Cohen.; Phys. Rev. B ,1994, 49, 5081
- X. Blasé.; A. Rubio.; S.G. Louie.; M.L. Cohen.; Europhys. Lett, 1994.28, 335.
- 52 .N.G. Chopra.; J. Luyken.; K. Cherry.; V.H. Crespi.; M.L. Cohen,S.G. Louie.; A. Zettl, Science.1995 , 269, 966
- N.G. Chopra.;A. Zettl.;Solid State Commun.1998 ,105, 297
- J. Cumings.; A. Zettl, Solid State Commun.2004, 129, 661
- R. Ma.; Y. Bando.; H. Zhu.; T. Sato, C. Xu, D. Wu, J. Am.Chem. Soc.2002, 124, 7672,
- P.W. Fowler, K.M. Rogers, G. Seifert, M. Terrones, and H. Terrones, Chem. Phys. Lett. 1999, 299, 359
- Monajjemi, M .; Falahati, M.; Mollaamin, F.; Ionics, 2013 , 19, 155–164
- Monajjemi , M.; Heshmat ,M.; Aghaei , H.; Ahmadi , R.; Zare,K. Bulletin of the Chemical Society of Ethiopia, 2007, 21 (1)
- Rubio, J.L. Corkill, M.L. Cohen, Phys. Rev. B.1994, 49,5081
- X. Blase, A. Rubio, S.G. Louie, M.L. Cohen, Europhys. Lett. 1994, 28 335.
- N.G. Chopra, J. Luyken, K. Cherry, V.H. Crespi, M.L. Cohen,S.G. Louie, A. Zettl, Science ,1995, 269, 966.
- O.R. Lourie, C.R. Jones, B.M. Bartlett, P.C. Gibbons, R.S. Ruoff,W.E. Buhro, Chem. Mater.2000, 12 ,1808;
- R. Ma, Y. Bando, T.Sato, Chem. Phys. Lett.2001, 337 ,61.
- W. Han, Y. Bando.; K. Kurashima.; T. Sato, Appl. Phys. Lett.1998, 73, 3085;
- (a) D. Golberg.; Y. Bando.; M. Eremets.; K. Takemura.; K. Kurashima.;H.Yusa, Appl. Phys. Lett.1996 ,69, 2045
- D.P. Yu, X.S. Sun, C.S. Lee, I. Bello, S.T. Lee, H.D. Gu, K.M. Leung, G.W. Zhou, Z.F. Dong.; Z. Zhang.; Appl. Phys. Lett.1998, 72 , 1966.
- F.Jensen.; H.Toftlund, Chem. Phys. Lett.1993, 201,89 , 94
- Monajjemi, M .; Sobhanmanesh, A .; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures,2013, 21 47–63
- Monajjemi ,M.; Karachi ,N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, ,2014, 22: 643–662
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.: Bull .Chem.Soc.Ethiop ,2008, 22(2),1-10.
- V. Tozzini.; F. Buda.; A. Fasolino.; physical review letters ,2000,21,85
- G. Seifert.; P. W. Fowler.; Mitchell, D.; Porezag, D.; Frauenheim, Th. Chem. Phys. Lett. 1997, 268, 252
- Mollaamin ,F.; Baei, MT.; Monajjemi, M.; Zhiani , R.; Honarparvar , B.; Russian Journal of Physical Chemistry A, Focus on Chemistry,2008, 82 (13), 2354-2361
- Monajjemi, M.; Ghiasi, R. ;Ketabi, S. Journal of Chemical Research.2004, 1: 11-18
- Mahdavian,L.; Monajjemi, M.; Mangkorntong ,N.Fullerenes, Nanotubes and Carbon Nanostructures,2009, 17 (5), 484-495
- Jon M. Matxain.; Jesus M. Ugalde.; M. D. Towler.; and R. J. Needs.; J. Phys. Chem. A 2003, 107, 10004-10010
- WU Haishun.;XU Xiaohong,; JIAO Haijun, ZHANG Fuqiang .; JIA Jianfeng. Chinese Science Bulletin. 2003, 48, 11 1102 1107
- Monajjemi, M .; Ketabi ,S.; Amiri, A. Russian Journal of Physical Chemistry , 2006, 80 (1), S55-S62
- M. Monajjemi .; Robert Wayne Jr, J.E. Boggs, Chemical. Physics. 433 (2014) 1-11
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F. Physics and Chemistry of Liquids,2008, 46 299.
- Mollaamin , F.; Varmaghani , Z.; Monajjemi , M, Physics and Chemistry of Liquids. 2011, 49 318
- Jon M. Matxain.; Jesus M. Ugalde.; M. D. Towler.; and R. J. Needs.; J. Phys. Chem. A 2003, 107, 10004-10010
- WU Haishun.; XU iaohong.; JIAO Haijun, ZHANG Fuqiang .; JIA Jianfeng Chinese Science Bulletin 2003, 48 (11), 1102 1107
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;. Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi ,M.; Mollaamin ,F. Journal of Computational and Theoretical Nanoscience,2012 ,9 (12) 2208-2214
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.; Honarparvar, B. Fullerenes, Nanotubes and Carbon Nanostructures, 2010, 18, 45–55
- Friedrich, B.; J.D. Weinstein.; R. Decarvalho .; J.M. Doyle.;. Trap. J. Chem. Phys. 1999, 110,2376-2383.
- Ramsay, N.E.; Magnetic Shilding of Nuclei. J. Phys. Rev.1950, 78, 699-703.
- Frischend, M.J.; J.B. Foresman, 1995. Gaussian 94 user’ reference (Gaussian, Inc., Pittsburgh).
- Ghalandari, B.; Monajjemi, M.; Mollaamin, F.; Journal of Computational and Theoretical Nanoscience, 2011 8, 1212–1219
- Monajjemi , M.; Khosravi , M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Tahan, A .; Monajjemi, M. Acta Biotheor, 2011, 59, 291–312
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Monajjemi, M.; Honarparvar, B.; H. Haeri, H.; Heshmat, M.; Russian Journal of Physical Chemistry C, 2008, 80(1),S40-S44.
- Cheeseman, J.R.; M.J. Frisch.; F.J. Devlin .;P.J.Stephens, Chemical Physics Letters, 1996,252 (3-4), 211-220.
- Pisani, C.;S. Casassa .; P. Ugliengo, Chemical Physics Letter. 1996, 253 (3-4) 201-208.
- Dresselhaus, M.; Dresselhaus, G.; Eklund, P. C., Science of Fullerenes and Carbon Nanotubes, San Diego: Academic Press, 1996, 109, 175.
- Oku,T.; Nishiwaki ,A.; Narita,I.; Gonda,G.. Chemical Physics Letters, 2003, 380, 620–623.
- Nirmala,V.; Kolandaivel,P.; Journal of Molecular Structure, 2007, THEOCHEM, 817,137-145.
- J.C. Charlier, J.C.; Rignanese,G.M. Phys. Rev. Lett, 2001, 86, 5970.

This work is licensed under a Creative Commons Attribution 4.0 International License.









