Heavy Metals monitoring in Psammotaea elongatafrom Semerak Lagoon, Kelantan, Malaysia
Hasrizal Shaari*1, Maidatul Suhana Mohd2, Nor Antonina Abdullah1 and Joseph Bidai2
1School of Marine and Environmental Science, University Malaysia Terengganu, 2Institute of Oceanography, University Malaysia Terengganu, 21030 Kuala Terengganu, Terengganu, Malaysia.
DOI : http://dx.doi.org/10.13005/ojc/310246
Article Received on :
Article Accepted on :
Article Published : 01 Jun 2015
Heavy metals concentrations in bivalve Psammotaea elongata from five sampling sites in Semerak Lagoon were determined by ICP-MS. The mean concentrations of Cd, Cu, Pb, Fe and Zn were 0.18, 4.42, 1.15, 1.47 and 46.35 µg/gin total soft tissue; 0.68, 20.08, 5.29, 85.5 and 9.06 µg/g in stomach; 0.7, 17.05, 4.39, 84.15 and 8.62 µg/g in leg; 0.74, 15.19, 4.4, 80.69 and 8.6 µg/g in ligament, respectively. The statistical ANOVA showed a significant difference between metals. Generally, metal concentrations in P. elongata were lower than the permissible limit suggests it is safe to be consumed by human.
KEYWORDS:Heavy metals; Psammotaea elongata, Lagoon
Download this article as:| Copy the following to cite this article: Shaari H, Mohd M. S, Abdullah N. A, Bidai J. Heavy Metals monitoring in Psammotaea elongatafrom Semerak Lagoon, Kelantan, Malaysia. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Shaari H, Mohd M. S, Abdullah N. A, Bidai J. Heavy Metals monitoring in Psammotaea elongatafrom Semerak Lagoon, Kelantan, Malaysia. Available from: http://www.orientjchem.org/?p=8964 |
Introduction
Heavy metals contamination on local, regional and global scales have been intensively studied in recent years due to the fact that of their persistency, toxicity, bioaccumulation in organisms, and risk to humans and ecosystems 1-2. This compound can be derived from natural or anthropogenic sources into aquatic environment. After discharge into the aquatic environment, heavy metals can reside as dissolved form in the water column or they can be removed from the water through sedimentation to the bottom sediments 3.
Bivalves as filter-feeding organisms enable to accumulate heavy metals in their body at much higher levels than those found in the water column, and for that reason they are used as bioindicator for the certain aquatic environments 2. However, this mechanism is strictly depending on the relationship between metal uptake and excretion, and the rate of diluting body growth of the organism 4. Bivalves Psammotaea elongata or Gari elongate are widely distributed in the western tropical region. P. elongata inhabits the intertidal sand banks or mud flats, lagoons and mangrove forest with sandy substrates 5. The bodies of this species are flattened from side to side and completely surrounded by two shells called valves. Interlocking two small cardinal teeth or sockets form the hinge on the valves, which are held together by an elastic ligament or fiber like tissue made up mostly of protein 5.
In this paper, we investigated the concentration of cadmium (Cd), cooper (Cu), lead (Pb), cobalt (Co) and zinc (Zn) in total flesh tissue and organs of P. elongata in Semerak Lagoon, Kelantan, Malaysia. We compared the concentrations of the studied metals with the other studies and the standard permissible limits. For the record, there is no studied have been carried out using this species in the study area.
Materials and Methods
Environmental Setting
Semerak Lagoon is semi-enclosed lagoon with a total area of 1.7 km2 located in the Pasir Puteh district, Kelantan (Figure 1). This lagoon is located at the edge of the Semerak River. The width of the lagoon is 200 to 800 m with an average depth of 3.12 m. Approximately 5,304,000 m2 water inside the lagoon during high tide 6.
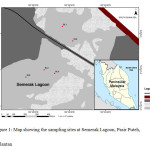 |
Figure1: Map showing the sampling sites at Semerak Lagoon, Pasir Puteh, Kelantan Click here to View figure |
Sample collection and preparation
The sampling was conducted in 2008. A total of 50 individuals of P. elongata were handpicked from five sampling sites during low tide (Table 1). Samples were packed in clean plastic bags and kept in cool icebox. The flesh of P. elongata was detached from the shell and separated to four subsamples, total soft tissue, stomach, ligament and leg. The samples were wet weighed and then freeze-dried at – 20 °C for 24 hours. The dried samples were finely ground and homogenized by ceramic mortar and pestle, and stored in the plastic vials before chemical analysis.
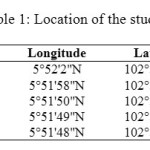 |
Table1: Location of the study sites |
Sample digestion and analysis
The chemical digestion was followed the established procedure by US EPA Method 200.3 (7) with some modification. Approximately 1g of homogenized sample was digested in the glass test tube with 10 mL HNO3 at 95 °C and left to reflux for 15 minutes. The similar procedure was repeated twice with 5ml HNO3 at 95 °C and left to reflux for 30 minutes. After evaporation to dryness, the residue was dissolved in 10 mL of H2O2 and diluted with mili-Q water. At this stage, a clear solution was obtained from complete digestion process. The digested sample was made up to 50 mL with 5 % HNO3. Analysis of the Cd, Cu, Pb, Co and Zn was performed using inductively coupled plasma mass spectrometry (ICP-MS). The method was verified via the certified reference material of Dogfish liver (DOLT-3). The analytical variance (ANOVA) was applied to understand the difference of studied metals concentrations between sampling stations and the different organ.
Results and Discussion
Heavy metals in total flesh tissue
The results of the standard reference material were in agreement with the certified values (Table 2). The recovery of the reference material were ranged from 95.95 to 102.24 % for all metals except Co, as no certified values was given for this metal.
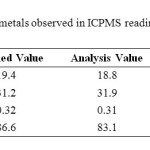 |
Table2: Recovery of metals observed in ICPMS reading using DOLT3 Click here to View table |
Concentrations of Cd, Cu, Pb, Co and Pb in total soft tissue of P. elongata from each sampling site are given in Table 3. The highest concentration of Cd was measured at SL1 at 0.17 µg/g, while the lowest concentration of it was measured at SL3 and SL5 at 0.19 µg/g. The highest concentration of Cu was measured at SL5 at 5.57 µg/g, while the lowest concentration of Cu was measured at SL2 at 3.25 µg/g. The highest concentration of Pb was measured at SL4 at 1.46 µg/g, while the lowest concentration of Pb was measured at SL3 at 0.86 µg/g. The highest concentration of Co was measured at SL5 at 1.57 µg/g, while the lowest concentration of Co was measured at SL3 at 1.39 µg/g. The highest concentration of Zn was measured at SL1 at 57.6 µg/g, while the lowest concentration of Zn was measured at SL2 at 37.58 µg/g. The average concentration of Cd, Cu, Pb, Co and Zn were 0.18±0.01 µg/g, 4.42±1.07 µg/g, 1.15±0.24 µg/g, 1.47±0.07 and 46.35±8.15 µg/g. In the total soft tissue, metals decreased in the order of Zn >Cu > Co > Pb > Cd
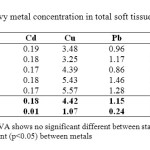 |
Table3: Heavy metal concentration in total soft tissue of P. elongate |
The conducted two-way ANOVA analysis indicated no significant difference (p < 0.05) of metals concentration in total soft tissue between sampling stations, but there was a significant difference (p > 0.05) between the studied metals (Table 3). This may due to the accumulated metals in P. elongata were differs with their own concentration and the uptake level. Philips (8) stated that the varying concentration of metals in tissues is due to the different effects of the different metal. The concentrations of heavy metals in the marine invertebrates, therefore, depend on the accumulation strategy adopted by species for different metal 4.
Zn was the highest metal concentration in total soft tissue and other organs. Some studies also revealed high concentration of Zn is occurs in the different bivalves species 9-10. According to George et al (11) bivalves have been considered as a strong Zn accumulator. Thus, this species may passively absorb Zn from surrounding area of its habitat (i.e. from seawater, sediment, and/or suspended particles). The inputs of Zn in this environment might be resulted from aquaculture activities 12. Normally, fish pellet that is used to feed the fishes in cage culture contains Zn and Cu as additional supplement 13. That is the reason why the concentration of Cu was also high in this study. Cu is an essential metal for the growth and life cycles of marine organisms including bivalve, but it become toxic at high concentration 14. Similar to Zn, this metal is probably derived from the aquaculture activities 15-16.
Furthermore, the mean concentration of Pb in the P. elongata was in agreement to the obtained value in the Soletellina sp. from nearby area of Tok Bali River 17. The existent of Pb in the environment is of concern because it is a cumulative poison in humans, and toxic to many aquatic organisms even at low concentrations 18. It has a long residence time compared with most other pollutants when released into the environment. This metal has low solubility and unable to be biodegraded by microbial activity.
Meanwhile, the concentrations of Co in the total soft tissue were relatively low throughout the sampling stations. There is no data of Co content in bivalves have been reported in Malaysia. Most efforts are focused on the distribution of this metal in soils and/or sediments. Naturally, small amounts of cobalt are found in water and animals. In context of this study area the existing of this metal may also derived from aquaculture activity because some cobalt elements (i.e. cyanocobalamin, cobalt carbonate and cobalt proteinate) are found in fish pellets 19.
Lastly, the concentration of Cd for all sampling stations was relatively lower compared to the other metals. This metal is widely distributed at low level in the environments 20. Although, Cd presented at very low level, but it may affects aquatic organisms 21. The major source of Cd in this environment is attributed to the use of fertilizersin agriculture 22. The input of agricultural-based Cd will assimilates with anoxic sediments of lagoon-bed that contain high organic matter 23, therefore, via filter-feed mechanism P. elongata absorb and accumulate this metal in their body.
Heavy metals in different organs
Concentrations of Cd, Cu, Pb, Co and Pb in the different organs of P. elongata from each sampling site are shown in figure 2. The studied metals concentration decreased in the different organs as same as that in the total flesh tissue. The concentrations of Cd in stomach, ligament and leg were ranging from 0.57 to 0.81 µg/g, 0.65 to 0.82 µg/g, and 0.55 to 0.80 µg/g. The mean concentrations Cd were 0.68±0.09 µg/g for stomach, 0.74±0.07 µg/g for ligament and 0.70±0.1 µg/g for leg. The concentrations of Cu in stomach, ligament and leg were ranging from 17.6 to 25.28 µg/g, 14.66 to 15.98 µg/g, and 14.28 to 18.2 µg/g. The mean concentrations of Cu were 20.08±3.16 µg/g for stomach, 15.19±0.59 µg/g for ligament and 17.0±1.6 µg/g for leg. The concentrations of Pb in stomach, ligament and leg were ranging from 4.33 to 6.60 µg/g, 3.85 to 5.06 µg/g, and 3.34 to 6.5 µg/g. The mean concentrations of Pb were 5.29±0.85 µg/g for stomach, 4.4±0.47 µg/g for ligament and 4.39±1.2 µg/g for leg. The concentrations of Co in stomach, ligament and leg were ranging from 8.82 to 9.22 µg/g, 8.35 to 8.81 µg/g, and 8.35 to 8.87 µg/g. The mean concentrations of Co were 9.06±0.17 µg/g for stomach, 8.6±0.19 µg/g for ligament and 8.62±0.24 µg/g for leg. The concentrations of Zn in stomach, ligament and leg were ranging from 78.75 to 90.0 µg/g, 54.68 to 92.50 µg/g, and 76.5 to 90.0 µg/g. The mean concentrations of Zn were 85.50±4.50 µg/g for stomach, 80.67±15.10 µg/g for ligament and 84.15±5.42 µg/g for leg.
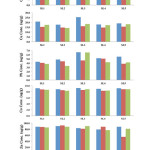 |
Figure2: Heavy metals in the different organ of P. elongata |
Statistical evaluation of one-way ANOVA showed a significant difference (p < 0.05) between mean heavy metals concentrations in the different organ. The metal concentrations in this species were varied may due to the different mechanism for metal binding and regulation 24, 25. The mean concentrations of Cu, Pb, Zn and Co were relatively higher in stomach than ligament, leg and total soft tissue. This is due to the filtered-food occurs in stomach than the other organ. Some also discovered high-accumulated heavy metals in digestive glands or stomach than the other organ of different bivalves species 26-28.
Comparison Metals Contents with Other Studies
Table 3 showed the comparison of the studied heavy metals with the other studies and guidelines on permissible limits. Heavy metals values detected in this species were relatively low as compared to the local 17, 24, 29, 30, 31 and some world studies 32-33. This result shows that the heavy metals level in bivalves in the Semerak Lagoon is still low compared to other locations. The value was almost similar with the bivalves in Poland 34,and Mexico 35. The present study also shows that the mean concentrations of heavy metals, Cd, Cu, Pb and Zn in P. elongata collected from the study site were found to be lower than the maximum permissible limits 36-37. Unlike for other elements, there are no limits are disclosed for Co in both standards.
From the observation, aquaculture activity is the most likely cause the loading of metals in this lagoon beside the input from the agriculture and urban area. We assume that the closed system setting of this lagoon may ease the metals transport from the aquaculture area through Semerak River during the high tide. Heavy metals that bind with particulate matter will sink on the lagoon-bed during low tide.
This study will provide scientific information on the contamination level and may be referred as initial report for future studies on the biota in this ecosystem. However, the results of this study are not sufficient to explain in detail about the other contaminant. Therefore, the continuous monitoring of heavy metals would provide an early signal of the intrusion of anthropogenic pollutant to this lagoon.
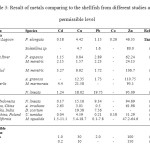 |
Table3: Result of metals comparing to the shellfish from different studies and the permissible level Click here to View table |
Conclussion
We presented the first data of heavy metals concentration in bivalve P. elongata and presumably reflected the heavy metals of Semerak Lagoon. The concentration of metals decrease according to the following order: Zn >Cu > Co > Pb > Cd. This study indicates that the study area is not contaminated with anthropogenic input, and this species tends to accumulate heavy metals that are lower than the permissible safety limits.
References
- Szefer, P; Frelek, K.; Szefer, K.; Lee, Ch-B.; Kim, B-S.; Warzocha, J.; Zdrojewska, I.; Ciesielski, T. Environ. Poll. 2002, 120, 423-444.
- Rainbow, P.S. Environ. Poll. 2002, 120, 497-507.
- Diez, S.; Lacorte, S.; Viana, P.; Barcelo, D.; Bayona, J.M. Environ. Poll. 2005 136, 525-536.
- Robert, W.F. and Philip S.R, Heavy metals in the marine environment; Boca Raton, CRC press. United States, (1990).
- Carpenter, K.E. and Niem, V.H. (eds) FAO species identification guide for fisheries purposes. The living marine resources of the Western Central Pacific, FAO, Rome, (1998).
- Arshad, M.A.; Alias, A.H.; Latun, A.R.; Siow, R. Impact of aquaculture activities in Semerak Lagoon, Kelantan. Under the “rehibilitation of habitats and biological diversity project”; Fisheries Research Institute, Penang, (2003).
- US EPA Method 200.3. Sample preparation procedure for spectrochemical determination of total recoverable elements in biological tissues, United States environmental protection agency environmental monitoring systems laboratory; Cincinnati, Ohio, (1991).
- Phillips, D.J.H. Quantitative Aquatic Biological Indicators: Their use to monitor trace metal and organochlorine pollution; Lond. Appl. Sci. Ltd. U.K., (1980).
- Eisler, R.; Barry, M.M.; Lapan, R.L.; Telek, G.; Davey, E.W.; Soper, A.E. Mar. Biol. 1978, 45, 311-317.
- Alam, L.; Mohamed, C.A.R.; Mokhtar, M.B. Sci. Asia. 2012, 38, 331-339.
- George, S.G.; Pirie, B.J.S.; Cheyne, A.R.; Coombs, T.L.; Grant, P.T. Mar. Biol. 1978, 45, 147-156.
- Casas, S.; Casas, D.; Gonzalez, J.L.; Bacher, C. Rapp. Comm. Int. Mer Médit. 2004, 37, 306-308.
- Javed, M. and Usmani, N. Global J Res Engg (C). 2012, 12, 27-34.
- Khaled, A. Egyptian J. Aqua. Biol. Fish. 2004, 8, 51-64.
- Cao, Z.H.; Hu, Z.Y. Chemophere. 2000, 41, 3-6.
- Riba, I., Blanco, J., Jimenez-Tenorio, N. and Del Valls, T.A. Chemosphere. 2005, 58, 659-669.
- Abdullah, N.A.; Shazili, N.A.M; Ahmad, A.S. Oriental J. Chem. 2009, 25(3), 477-484.
- Hart, B. Hydrobiologia. 1982, 91, 299-313.
- Naylor, S.J.; Moccia, R.D.; Durant, G.M. N. Am. J. Aquacult. 1999, 61(1), 21-26.
- FDA. Fish and Fisheries Products Hazards and Controls Guidance-Fourth Edition. Centre for Food Safety and Applied Nutrition, US Food and Drug Administration, (2011).
- Kennish, M.J. Ecology of Estuaries: Anthropogenic Effects. Boca Raton, CRC press. USA, (1992).
- Louekari, K.; Mäkelä-Kurtto, R.; Virtanen, J.P.V.; Sippola, J.; Malm, J. Cadmium in fertilizers. Risk to human health and the environment. Ministry Agriculture and Forestry. Finland. Helsinki, (2000)
- Muniz, P., Danulat, E., Yanicelli, B., García-Alonso, J., Medina, G. and Bícego, M.C. Environ. Int. 2004, 29, 1019-1028.
- Mohamad Yusof, N.A.; Shabdin, M.L. UMTAS 2011. 2011, 806-811.
- Li, Y.; Yu, Z.; Song, X.; Mu, Q. Environ. Monit. Assess. 2006, 121, 491-501.
- Yap, C.K.; Tan, S.G.; Ismail, A.; Omar, H. Environ. Int. 2004, 30, 39-46.
- Balachandra, W.; Satish, S. Biology. 2013, 3 (4), 10-13.
- Berandah, F.E.; Yap, C.K.; Ismail, A. Environ. Asia. 2010, 3(1), 65-71.
- Abdullah, M.H.; Sidi, J.; Aris, A.Z. Int. J. Environ. Sci. Edu. 2007, 2 (3), 69-74.
- Mohd Zahir, M.S; Kamaruzzaman, B.Y.; Akbar John, B.; Jalal, K.C.A.; Shahbudin S.; Al-Barwani, S.M.; Goddard, J.S. Oriental J. Chem. 2011, 27(3), 979-984.
- Amin, B.; Ismail, A.; Arshad, A.; Che, K.Y.; Kamarudin, M.S. J. Coast. Dev. 2006, 10 (1), 19-32.
- Li, P.; Gao, X. Ecotox. Environ. Safe. 2014, 109, 1-9.
- Singh, Y.T.; Krishnamoorthy, M.; Seetharamaiah, H.; Thippeswamy. IJRRPAS. 2013, 3(2), 254-267.
- Rzymski, P.; Niedzielski, P.; Klimaszyk, P.; Poniedziałek, B. Environ. Monit. Assess. 2014, 186, 3199-3212.
- Mendez, L.; Palacios, E.; Acosta, B.; Monsalvo-Spencer, P.; Alvarez-Castaneda, T. Biol. Trace Elem. Res. 2006, 110, 275-287.
- FAO/WHO, List of maximum levels recommended for contaminants by the Joint FAO/ WHO codex alimentarius commission, second series. CAC/FAL, Rome. 1984, 3, 1-8.
- Malaysian Food and Regulations (MFR), In Hamid Ibrahim, Nasser and Yap Thiam Huat. Malaysian Law on Food and Drugs; Malaysia Law Publisher, Kuala Lumpur (1985).

This work is licensed under a Creative Commons Attribution 4.0 International License.









