Green synthesis and dye propertied of 2H-indazolo [2, 1-b] phthalazinetriones by PEG-MDIL
Bijan Mombani Godajddr,* Masoumeh Etamad, Soghra Soliemani
Department of Chemistry, Islamic Azad University Omidiyeh Branch, Omidiyeh, Iran
Email: bmombini@gmail.comDOI : http://dx.doi.org/10.13005/ojc/310283
Article Received on :
Article Accepted on :
Article Published : 12 Jun 2015
An efficient one-pot condensation of aldehyde, dimedone, and phthalhydrazide has been achieved in the presence of a catalytic amount of PEG-MDIL as a novel environmentally benign magnetic ionic catalyst under solvent-free conditions. This methodology illustrates a very simple procedure, with wide applicability, environmental friendliness and reusability of catalyst. The new catalyst was characterized using some different spectroscopic techniques such as FT-IR and UV spectroscopy. The novel catalyst has therefore a great potential to be used in green processes.
KEYWORDS:One-pot; dimedone; phthalhydrazide; multicomponent reaction
Download this article as:| Copy the following to cite this article: Godajddr B. M* Etamad M, Soliemani S. Green synthesis and dye propertied of 2H-indazolo [2, 1-b] phthalazinetriones by PEG-MDIL. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Godajddr B. M* Etamad M, Soliemani S. Green synthesis and dye propertied of 2H-indazolo [2, 1-b] phthalazinetriones by PEG-MDIL. Available from: http://www.orientjchem.org/?p=9241 |
Introduction
Heterocyclic compounds occur very widely in nature and are essential to life. Among a large variety of heterocyclic compounds, heterocyclic containing phthalazine moiety are of interest because they show some pharmacological and biological activities. Phthalazine derivatives were reported to possess anticonvulsant, cardiotonic, and vasorelaxant activities.6-8 Therefore, a number of methods have been reported for the synthesis of phthalazine derivatives.1-9
Solvents are chemical substances used in huge amounts for many different applications. In many cases, organic solvents are chemical substances derived from petrol, and have a negative impact on the health and the environment. One of the key areas of green chemistry is the elimination of solvents in chemical processes or the replacement of hazardous solvents with environmentally benign solvents.18The possibility of performing multicomponent reactions under solvent-free conditions with a homogeneous catalyst could enhance their efficiency from economic and ecological points of view.
The aim of this presented protocol is to highlight the synergistic effects of the combined use of MCRs and reactions under solvent-free conditions with a homogeneous catalyst for the development of a new eco-compatible strategy for the synthesis of heterocyclic compounds.
Experimental
Material and Methods
Melting points were measured on an Electro thermal 9100 apparatus and are uncorrected.1H &13CNMR spectra were recorded on a Bruker Advanced DPX 400 MHz instrument spectrometer using TMS as the internal standard in CDCl3. IR spectra were recorded on a BOMEM MB-Series 1988 FT-IR spectrometer. Phtalhydrazide, dimidone and PEG-600 were purchased from Merck Company in high purity. Products were characterized by comparison of their physical and spectroscopic data with those of known samples. The purity of products and reaction monitoring was accomplished by TLC on silica gel Poly GramSILG/UV 254 plates.
The synthesis of dichloro substituted PEG-600 (Cl-PEG-Cl)
PEG-600 (15 g, 0.025 mol) and pyridine (5 mL, 0.0625 mol) was dissolved in toluene (20 mL), stirred at 87 ◦C, thionyl chloride (5mL, 0.0625 mol) as added slowly, and the resultant reaction mixture was stirred for 15 h at 87 ◦C. Then the resulting solid was removed by filtration. After removal of the solvent under reduced pressure a viscous liquid residual was collected as dichloro substituted PEG-600 (13.75 g, 91.6 %).
Procedure for preparation of Poly (ethylene glycol) bis (methylimidazolium dichloride) (PEG-DIL)
1-Methylimidazole (2 mmol), and polyethylene glycol dichloride (1 mmol) were placed in a Pyrex glass tube, sealed and heated at 80 °C for 16 h or 110 °C for 20 h, respectively. The organic solvent was removed and extracted with ethyl acetate (3 × 20 mL) which was then washed with water (2 × 20 mL) and ether (2 × 10 mL), and dried over anhydrous magnesium sulfate. The solvent was removed under vacuum at 65 °C overnight to give colorless product in high yield (85 %). 1H NMR (400 MHz, CDCl3, ppm): δ H= 3.58 (44H, m,O–CH2–CH2–O), 3.82 (4H, t, O–CH2–CH2–N (, 4.01(6H, s, N–CH3), 4.54 (4H, t, N–CH2–CH2–O), 7.74, 7.60(4H, C(4,5)–H), 9.68 (2H, C(2)–H).
Procedure for preparation of PEG-MDIL
PEG-MDIL, was prepared by mixing PEG-DIL (1 mmol) with anhydrous FeCl3 (2 mmol) at room temperature for 3h, a dark brown liquid was obtained. The obtained PEG-MDIL was extracted with small amount of ethyl acetate. The solvent was evaporated and the resulting clear brown liquid was dried in vacuum oven at 60 °C for 24 h. The PEG-MDIL was obtained in high yield (87 %). Elem. Anal.
Calc. for C34H64N4O12Cl8Fe2: C, 51.58; H, 8.09; N, 7.07. Found: C, 49.75; H, 7.81; N, 6.62 %.
Synthesis of 2H-indazolo [2, 1-b] phthalazine-triones derivatives: General Procedure
A mixture of dimedone (0.14 g, 1 mmol), phthalhydrazide (0.16 g, 1 mmol), an aromatic aldehyde (1.1 mmol) and PEG-MDIL (0.1 g) was heated at 100 °C for 10 min. Completion of the reaction was indicated by TLC. After completion of the reaction, water (20 mL) was added to the mixture, and extracted product the insoluble crude product was dissolved in by ethyl acetate (3×10 mL). The organic phase was separated and evaporation of the solvent under reduced pressure. The solid product recrystallized from ethanol to afford the pure product 4a-l.
3, 4-Dihydro-3, 3-dimethyl-13-phenyl-2H-indazolo [2, 1-b] phthalazine-1, 6, 11(13H)-trione(4a). Yellow powder; M. P.: 205-207 °C; IR (KBr, cm–1): 2958, 1662, 1576; 1H-NMR (400 MHz, CDCl3, δ / ppm): 1.25 (6H, s, 2Me), 2.36 (2H, s, CH2CO), 3.24 and 3.45 (2H, AB system, J = 18.6 Hz, CHaHbCO), 6.48 (1H, s, CHN), 7.32– 8.36(9H, m, Ar-H); C-NMR (100 MHz, CDCl3, δ / ppm): 28.2, 28.6, 34.7, 38.2, 50.8, 64.7, 118.5, 127.2, 127.8, 127.9, 128.8, 128.7, 129.2, 133.6, 134.2, 136.5, 150.9, 154.4, 156.2, 192.2.
3, 4-Dihydro-3, 3-dimethyl-13-(4-methoxyphenyl)-2H-indazolo [2, 1-b] phthalazine-1, 6, 11(13H)-trione(4b). Yellow powder; M. P.: 220-222 °C;IR (KBr, cm–1) 2959, 1655, 1626; 1H-NMR (300 MHz, CDCl3, δ / ppm): 8.24–8.35 (2H, m), 7.82–7.85 (2H, m), 6.84–7.33 (4H, m), 6.43 (1H, s), 3.77 (3H, s), 3.22 and 3.41 (2H, AB system, J = 19.2 Hz), 2.34 (2H, s), 1.22 (6H, s); 13C-NMR (100 MHz, CDCl3, δ / ppm): 192.02, 159.74, 156.07, 154.27, 150.74, 134.46, 133.47, 129.17, 128.96, 128.41, 128.26, 127.83, 127.72, 118.47, 114.24, 64.58, 55.31, 50.98, 38.06, 34.64, 28.80, 28.41.
Results and Discussion
Here in, a straightforward convergent one-pot synthesis of 2H-indazolo[2,1-b]phthalazine-trione derivatives using PEG-MDIL as an efficient magnetic ionic liquid catalyst under solvent-free conditions through the domino Knoevenagel condensation/Michael addition /intramolecular cyclo-dehydration sequence was examined.
In continuation of these studies, we have found that PEG-MDIL as a newly reported reagent (Scheme 1).10 All reactions are performed under mild reaction conditions in good to high yields.
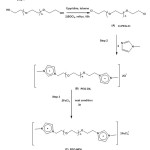 |
Scheme1: Synthesis of PEG-MDIL |
To evaluate the catalytic activity of PEG-MDIL in the preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives, a model three-component coupling reaction of phthalhydrazide (1 mmol), dimedone (1 mmol) and benzaldehyde (1.1 mmol) under solvent-free conditions at 100 °C in the absence and presence of PEG-MDIL were examined.
In order to be able to carry out preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H)-trione derivatives in a more efficient way minimizing the time, temperature and amount of catalyst, the reaction of benzaldehyde, phthalhydrazide and dimedone was selected as model system to the effects of the catalyst at different reaction temperatures (25, 60, 80, 100 and 120 °C and the different amount of catalyst (0, 0.05, 0.1, 0.15, and 0.2 g) were investigated. The reaction using 0.1 g of PEG-MDIL at 100 °C proceeded in highest yield. Further increase in temperature to, 100 °C had little effect on the rate of reaction. Therefore, we kept the reaction temperature at 100 °C as optimal temperature.According to Table 1, this reaction was carried out without catalyst under solvent free conditions in order to establish the effectiveness of the catalyst. It was found that 2H-indazolo [2, 1-b] phthalazine-1, 6, 11(13H)-trione was not made after 1h of heating.
1.2: 1:1 ratio of benzaldehyde, dimedone, phthalhydrazide and 0.1 g of PEG-MDIL after 10 min at 100 °C.
After optimizing the conditions, the generality of this process was demonstrated by the wide range aryl aldehydes to synthesize the corresponding products in excellent yields (Scheme 2, Table 2). As seen from Table 2, aromatic aldehydes having electron-donating as well as electron-withdrawing groups were uniformly transformed into the corresponding products in high yields within 10-15 min. Substituent on the aromatic ring had no obvious effect on yield or reaction time under the above optimal conditions. Unlike some previously reported methods, the present method does not require toxic organic solvents to produce the 2H-indazolo [2, 1-b] phthalazine-1, 6, 11(13H)-trione derivatives.
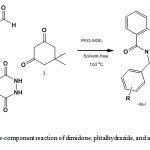 |
Scheme2: Three-component reaction of dimidone, phtalhydrazide, and aromaticaldehyde Click here to View scheme |
In view of the above experimental facts, we propose a tentative mechanism for this multi component reaction which is depicted in Scheme 3. Initially the counter-cation BMIMPEG2+ in the ionic liquid, PEG-MDIL, acts as a Lewis acid, catalyze Knoevenagel condensation between the aldehyde and dimedone to generate adduct A, which acts as Michael acceptor. The phthalhydrazideattacks adduct A in a Michael-type fashion to produce an open chain intermediate B. Intermediate B undergoes intramolecular cyclization by the reaction of nucleophilic amino function cation BMIMPEG2+ in the ionic liquid, PEG-MDIL, acts as a Lewis acid, catalyze Knoevenagel condensation between the aldehyde and dimedone to generate adduct A, which acts as Michael acceptor. The phthalhydrazideattacks adduct A in a Michael-type fashion to produce an open chain intermediate B. Intermediate B undergoes intramolecular cyclization by the reaction of nucleophilic amino function to carbonyl group followed by dehydration to form 2H-indazolo[2,1-b]phthalazine-triones.
Table1: Synthesis of 2H-indazolo [2, 1-b] phthalazinetrione derivatives
|
M.P./ °C |
Yield (%) |
Time (min) |
R |
Product |
Entry |
|
|
ReportedRef] |
Found |
|||||
|
204-2063 |
205-207 |
89 |
10 |
H |
4a |
1 |
|
218-2209 |
220-222 |
90 |
15 |
4-OCH3 |
4b |
2 |
|
226-23111 |
228-230 |
90 |
10 |
4-CH3 |
4c |
3 |
|
266-2699 |
264-266 |
86 |
15 |
2-Cl |
4d |
4 |
|
262-2643 |
262-265 |
87 |
10 |
4-Cl |
4e |
5 |
|
269-2713 |
268-270 |
88 |
15 |
3-NO2 |
4f |
6 |
|
218-22011 |
220-222 |
87 |
15 |
2,4-Cl2 |
4g |
7 |
|
265-26712 |
262-264 |
89 |
15 |
4-Br |
4h |
8 |
|
220-2249 |
221-224 |
87 |
10 |
4-F |
4i |
9 |
|
216-2183 |
222-224 |
85 |
15 |
4-NO2 |
4j |
11 |
|
213-21512 |
211-213 |
89 |
10 |
3-CF3 |
4k |
12 |
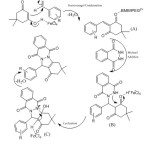 |
Scheme3: A plausible reaction mechanism |
Conclusion
In conclusion, we have successfully developed a simple and green catalytic procedure for the efficient synthesis of phthalazine-triones using PEG-MDIL and under mild reaction conditions. PEG-MDIL can replace the ILs and other homogeneous catalysts with reasonable recovery and reusability and therefore suitable for industrial applications.
Acknowledgments
We gratefully thank Islamic Azad University, Science and Research Branch Omidiyeh for financial support.
References
- Ryu CK, Park R E, Ma M Y, NhoJ H Bioorg. Med. Chem. Lett. 2007.,17 2577.
- Fazaeli R, Aliyan H, Fazaeli N Open Catal. J.,2010,.3 14.
- Khurana J M, Magoo D Tetrahedron Lett. 2009.,50.,7300.
- Vaghei R G, Karimi‐Nami R, Toghraei‐Semiromi Z, Amiri M, Ghavidel M 2011 Tetrahedron, 67 1930.
- Shukla G, Verma R K, Verma GK, SinghM S Tetrahedron Lett.,2011,52.,7195.
- Shekouhy M, Hasaninejad A Ultrasonics Sonochemistry, 2012 .,19.,307.
- Mosaddegh E, Hassankhani A Tetrahedron Lett. 2011., 52.,488.
- Nagarapu L, Rajashaker B, Hari Babu M J. Heterocyclic Chem. 2009.,48., 728.
- Shaterian H R, Ghashang M, Feyzi M Appl. Catal.A: Gen2008,.345.,128.
- (a) Godajdar B M, Ansari B, 2015 J. Mol. Liq. 202 34.(b) Godajdar B M, Kiasat A R, Hashemi M M 2013J. Mol. Liq.183, 14; (c) Godajdar B M, Kiasat AR, Hashemi M M 2013 Heterocycles, 87 559; (d) GodajdarB M,Kiasat A R 2013J. Chil. Chem. Soc.58 1850; (e) Godajdar B M, SoleimaniS 2014 J. Chin. Chem. Soc. 61 447.
- Shaterian H R, Hosseinian A, Ghashang M Arkivoc 2009.,2 59.
- Wang H J, ZhangX N, Zhang Z H Monatsh. Chem. 2010.,141., 42

This work is licensed under a Creative Commons Attribution 4.0 International License.









