Electrophoretic Studies on the Complexation of Pb(Ii) and Cd(Ii) with Chloramphenicol Drug
Sarla Tripathi, Arvind Singh, Pradeep Mishra and O. P. Rai*
Department of Chemistry, Govt. (Autonomous) P. G. College, Satna (M.P.) 485001, India
DOI : http://dx.doi.org/10.13005/ojc/310247
Article Received on :
Article Accepted on :
Article Published : 21 Apr 2015
The complexation reactions of Pb(II) and Cd(II) with chloramphenicol drug in solution were studied by paper electrophoretic technique. The Stabilty constant of Pb(II) – Chloramphenicol and Cd(II) – Chloramphenicol binary complexes have been found to be 3.26 and 3.07 (logarithm stability constant values), respectively at 25°C temperature and 0.1M (HClO4) ionic strength.
KEYWORDS:Paper electrophoresis; Pb(II); Cd(II); Chloramphenicol; Chelation; Binary complexes; Stability constants
Download this article as:| Copy the following to cite this article: Tripathi S, Singh A, Mishra P, Rai O. P. Electrophoretic Studies on the Complexation of Pb(Ii) and Cd(Ii) with Chloramphenicol Drug. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Tripathi S, Singh A, Mishra P, Rai O. P. Electrophoretic Studies on the Complexation of Pb(Ii) and Cd(Ii) with Chloramphenicol Drug. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8526 |
Introduction
Metal ions play an important role in biological system and required for many critical functions in human1-3 but Pb and Cd are classified as toxic metal by Banerja4. Toxic metals are not bio-degradable and their persistance in environment leads to accumulation in living organism causing serious health problems in plants, animals and human beings. The chelation therapy for intoxication of metals depends upon the chelating agents being able to reach the intercellular site where the metals are firmly bound. For a pretty long time, varieties of ligand have been used as chelating agent to combat metal poisoning. In the present work Chloramphenicol [2, 2-dichloro-N-{(1R, 2R)-2-hydroxy-1-(hydroxymethyl)-2-(4 nitrophenyl)ethyl}-acetamide] a bacteriostatic-antibiotic with formula C11H12Cl2N2O5 having molecular mass 323.1320 g/mol has been used as ligand. A search of the Literature5-10 indicated that no report is available on Pb(II) and Cd(II) binary complexes with chloramphenicol. This paper describes a paper electrophoretic method for the determination of stability constant of these complexes in solution. Stability constant is useful physical entity, which explains the importance and function of various complexes in biological systems. Thus useful information may be obtained from stability data relating to metal ions and ligand. A significant development on the determination of stability constants of complexes was made by Jokl11 in 1964. A theoretical treatment similar to that of Jokl was adopted by Biernet12 for the step wise complex formation.
Experimental
Chemicals
Metal perchlorates were prepared by precipitation of metal carbonates from 0.1 M solution of Chlorides of Pb(II) and Cd(II) (A.R. grade) with the solution of sodium carbonate. The precipitate were thoroughly washed with boiling water and treated with a calculated amount of 1% (AR grade) perchloric acid. These were boiled on a water bath and filtered. The metal contents of the filtrate were determined and final concentration kept at 5.0 × 10-3 M. The solution were standardized and diluted with distilled water as required. Metal spots were detected on the filter paper using 0.1% (W/V) solution of 1–(2-pyridylazo)–2-naphthol (PAN) in ethanol was used for detecting the Pb(II) and Cd(II) metal ions. 5.0 × 10-3 M glucose (BDH A. R. grade) was prepared in water and used as an electro–osmotic indicator for the correction due to electro–osmosis. A saturated aqueous solution (0.9 ml) of silver nitrate was diluted with acetone to 20 ml. Glucose was detected by spraying with this solution, and then with 2% ethanolic sodium hydroxide when a black spot was formed.
Background Electrolyte
Stock solution of (5.0 M) was prepared by suitable dilution of 70% perchloric acid (SDS A. R. grade). 2.0 M sodium hydroxide (A. R. grade) and 0.5 M stock solutions of the complexing reagent Chloramphenicol (A. R. grade) solutions were prepared. All chemicals were used without further purifications. Each solution was standardized using the appropriate method. The background electrolyte used in the study of binary complexes was 0.1 M perchloric acid and 0.01 M chloramphenicol. The system was maintained at various pH by the addition of sodium hydroxide.
Instrument and Procedure
A Systronic (Naroda, India) Model 610 electrophoresis system was used. The details of apparatus and procedure adopted in present study are described in literature13-14.
Electrophoretic observations on metal ion spots were recorded at various pH values of the background electrolyte obtained by adding sodium hydroxide solution, the ionic strength being maintained at 0.1 M. The observed mobility of the migrant was calculated by using the formula.
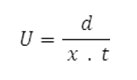
After applying the correction factor, the observed mobility is given as
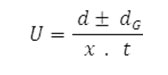
Where U = mobility of metal ion/complex ion, d = mean of duplicate distances travelled by metal ion/complex ion, dG = mean of duplicate distances travelled by glucose spots, x = field strength and t = time for electrophoresis.
The electrophoresis was carried out for 30 minutes as for metal ions. The mobility of the metal ion spots on the strips were reported along with pH values. The individual mobility of the duplicate spots was fairly equal and the variation was always less than 0.5 cm.
Result and Discussion
The electrophoretic mobility of metal ion spot against pH gives number of plateaus shown in figure 1.
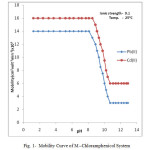 |
Figure1: Mobility Curve of M –Chloramphenicol System |
A plateau is obviously show pH range where mobility is practically constant. In the region of first plateau metal ions are uncomplexed. It lies in low pH region where concerned of highly protonated species of ligand is obviously maximum, hence it is concluded that this protonated species of ligand is not complexing. Beyond this pH range metal ion spot has progressively decreasing mobility. This decrease shows formation of complex of metal ion with ligand. A point is reached beyond which mobility of the metal ion species remain constant. This is the second plateau which corresponds to a pH region in which 1:1 cationic complex is formed. One ligand anion combines with each metal ion to form [Mn(L)]+, cationic complexes with Pb(II) and Cd(II) respectively. On further increase of pH beyond the second plateau there is no further decrease in mobility of metal ion indicates no further complexation takes place. It is significant that these studies give clear evidence of the binary complex formation of 1:1 composition. The complexation of metal ions with ligand anion [L–] can be represented as
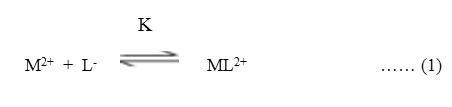
Where M2+ = Pb2+ and Cd2+ metal ion,
[L–] = chloramphenicol anion,
K = stability constants, respectively.
The metal spot on the paper is thus a combination of uncomplexed metal ions and 1:1 complex. The spot is moving under the influence of electric field the overall mobility can be given by equation.
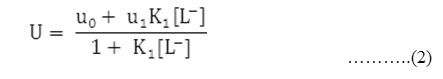
Where u0 and u1 are the ionic mobilities of uncomplexed metal ions and 1:1 metal complexes, respectively.
Accordingly the pH corresponding to the average value of u0 and u1 is found from the figures and with the knowledge of dissociation constant of chloramphenicol(pka =11.03). the concentration of ionic ligands at this pH is calculated. Its reciprocal gives the stability constant K of the 1:1 complex. The concentration of chelating ligands anion [L–] is calculated from equation,
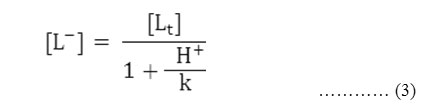
Where [Lt] = total concentration of the ligand chloramphenicol (0.01 molL-1 )
k = dissociation constant of chloramphenicol, respectively.
The stability constant
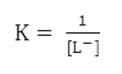
The calculated stability constants value for 1:1 binary complexes are given in table 1 which follows the Irving William’s15 order for stability constants of transition metals of the first transition series and no comparison can be made with the literature value due to its unavailability.
Table1: Stability Constant of M – Chloramphenicol Binary Complexes
Temperature – 250C Ionic Strength – 0.1M
| Metal ions |
Stability Constants |
|
|
Calculated Values logK |
Literature values logK |
|
|
Pb(II) |
3.26 |
– |
|
Cd(II) |
3.07 |
– |
Conclusion
Metal toxicity is a major problem for human and other living beings. Pb(II) and Cd(II) are significant for biological systems but as such they are toxic. Chelating agents and stability constant are important parameter for formation of metal complexes. Chloramphenicol used in this work is very good chelating agents which interacted with metal ions to form various metal chelates. It can be concluded from the present study that chloramphenicol may be used to reduce the levels of Pb(II) and Cd(II) in biological system.
References
- Beck, M.T., The determination of complex equilibria. Chapter 8, Van Nostrand, New York (1969).
- Alimarin, I. P. and Sheleskaya, V. I., Pure Appl. Chem. 1970., 21, 461
- Janes, D. L. and Margerum,W. M., Inorg. Chem., 1966. 5, 1135
- Banerja, D., Everyman’s Sci., 1995. 29, 176
- Tewari, B. B., Journal of Chromatography A, 2002. 962, 233-237
- Tewari, B. B., J. Mex. Chem. Soc., 2008.,52(3), 219-223
- Tewari B. B., Revista Bolivina De Quimica., 2008.,25(1), 4-9
- Tewari B. B., Revista Bolivina De Quimica., 2013. 30(2), 153-155
- Sachan, S. et al, Croat. Chem. Acta, 2011. 84(4), 461-464
- Singh, P. P. and Kanaujia, S., Chem. Sci. Trans., 2013.,2(3), 1028-1034
- Jokl, V. J., Chromatography, 1964., 14, 74.
- Biernet, J., Rocz. Chem., 1964. 38, 343
- Singh, A. and Rai, O. P., Orient. J. Chem., 2014.30(4), 2059-2063
- Singh, A., Patel, R. R. and Rai, O. P., J. Chem. & Cheml. Sci. 2014, 5(1), 29-35
- Irving, H. and Williams, R. J. P., Nature, 1948. 162, 746

This work is licensed under a Creative Commons Attribution 4.0 International License.









