An experimental study of formation of the mercury mixed halides HgClBr and HgBrI and of their purity
Rabia Ahmad*, Jamshed Ali and Qamer Faisal
1Department of Chemistry, Faculty of Natural Sciences, Jamia Millia Islamia, (Central University), Jamia Nagar, New Delhi 110025, India.
DOI : http://dx.doi.org/10.13005/OJC/310206
Article Received on :
Article Accepted on :
Article Published : 03 May 2015
Claims to have produced the mixed halides of mercury are very old. However, their stability or even their very existence was seriously questioned by Ammlung and Brill several decades back, on the basis of their study, in several solvents, of what was thought to be HgBrI. The mixed halide HgClI was already known to be unstable. On the basis of these facts, which were also lent some theoretical support, it was strongly conjectured that the mixed halides of mercury and similar elements, were expected to be unstable. However, the matter does not seem to have received the attention it deserved. It was in this light that this study was taken up. What has been thought to be HgClBr has been produced by several methods and HgBrI by one or rather two methods. The product has been subjected to X-ray diffraction, FTIR and Raman studies. Studies confined to the solid product are being reported here and only those results are being presented for which all the three techniques could be employed. These studies show that a new product is indeed formed in most of these cases, but the product is not pure in any of these cases, although the impurity seems to be quite small in most of these cases. This calls for having a thorough look at not only the mixed halides of the elements, but of all compounds claimed to be like:
KEYWORDS:Mixed halides; impure product; d-values; FTIR Studies
Download this article as:| Copy the following to cite this article: Ahmad R, Ali J, Faisal Q. An experimental study of formation of the mercury mixed halides HgClBr and HgBrI and of their purity. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Ahmad R, Ali J, Faisal Q. An experimental study of formation of the mercury mixed halides HgClBr and HgBrI and of their purity. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8762 |
Introduction
Mercury has been known for a long time and its compounds studied1. Claims to have produced mixed halides of mercury go fairly long back into time2. However, Ammlung and Brill and others have thoroughly studied the problem and these authors have seriously challenged existence of the mixed halides3, 4. Ammlung and Brill carried out a study of the Raman lines of what was claimed to be HgBrI, dissolved in some solvents. They found strong Raman lines of the constituent di-halides of mercury3. The mixed halide HgClI had already been found to be highly unstable. Some theoretical support to this idea was also indicated.
However, rather surprisingly, almost no notice of Ammlung and Brill’s work seems to have been taken for quite a long time. Workers continued to claim formation of the di-halides of not only mercury but also of cadmium and zinc5,6. Claims have been made of determination of structures of mixed mercury halide crystals7. Nobody seems to have thoroughly investigated the extent of the correctness of these claims.
We seem to be the first workers to have thoroughly investigated what seems to be HgClBr and to some extent HgBrI also. The main reason was that Ammlung and Brill had confined their study only to what was thought to be HgBrI dissolved in some solvents. We have extended our study to not only what would be regarded as HgBrI but much more extensively to what would be regarded as HgClBr. Besides considering many more solvents, we have additionally studied the product in the solid state. This work is concerned only with the product mixed halide in the solid state.
We briefly mention the different ways in which what is regarded as HgClBr has been prepared, using the methods suggested in the literature as well as some of our own. Earlier some X-ray studies of the products formed by these methods have been taken up by some authors8.
We have produced what would be regarded as HgClBr by several methods and have also produced what would be regarded as HgBrI by two methods. All these methods are given below.
Method –I
By heating an equmolar mixture of HgCl2 and HgBr2 in an oven at 80°C, for 48 hours (The same reaction has also been carried out at 100°C and 150°C for 10 hours in each case).
HgCl2 + HgBr2 → HgClBr
Method –II
In the method of preparation, a saturated solution of HgCl2 and HgBr2 in water mixed in 1:1 ratio, was kept in a desiccator over CaCl2 until solid crystals separate out
HgCl2 + HgBr2 → 2HgClBr
HgClBr produced by this method is in nanoform9. Several methods are available for grain size measurementse.g.10. However, we have used the Debye-Scherer formula11.
Method – III
In the method due to R.P. Rastogi, bromine gas is passed over solid mercurous chloride until a constant weight is obtained7
Hg2Cl2 + Br2 → 2HgClBr
Preparation of HgBrI
Method – I
HgBrI was prepared by mixing equimolar solutions of HgBr2 and HgI2 in acetone. Crystals of HgBrI separated after evaporation
HgBr2 + HgI2 → 2HgBrI
Method-II
An equimolar mixture of HgBr2 and HgI2 is taken and then to grind the two halides together at room temperature. This is called Type-II mixture.
Type I and Type II mixture and use of the X-ray d values for identification of a new product.
X-ray d-values are extensively used for identifying the compounds present in a mixture. The standard practice is to locate the three most prominent peaks of the compound for its identification. The most important matter is to decide how much deviation in the d-values is permissible. This is what we find out.
Extension of this approach for concluding if a new product has been formed in a reaction is direct and straightforward. This is discussed here.
One needs the diffractogram of the constituent reactants and additionally the diffractogram of an equimolar mixture of the reactants, when they are separately ground and then added to each other, is very helpful. This type of equimolar mixture may be called the Type-I mixture. We discuss a simple case as an illustration.
We have taken the diffractograms of HgCl2, HgBr2 and of their Type-I mixture. These are given in Figs. 1, 2 and 3 respectively. We prepare the equimolar mixture in another way also. We take suitable equimolar amounts, mix these and grind these in a mortar. We call it the Type-II mixture. The diffractogram is given in Fig. 4. The d-values for the above cases are given in Tables 1-4, respectively.
 |
Figure1: HgCl2 |
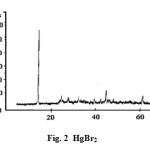 |
Figure2: HgBr2 Click here to View figure |
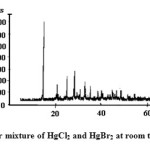 |
Figure3: Equimolar mixture of HgCl2 and HgBr2 at room temperature (Type I) Click here to View figure |
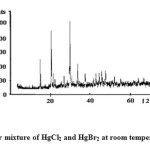 |
Figure4: Equimolar mixture of HgCl2 and HgBr2 at room temperature (Type II) Click here to View figure |
One would expect no chemical or even physical change to have occurred in the Type I mixture. Therefore, it may be taken to give the shifts in the d-values that would be expected to occur in a mixture of these compounds. Greater shifts from those noted here would be regarded as belonging to a new compound. A totally new peak would be an unmistakable signal for the formation of a new compound. Occurrence of peaks of the reactants within the permissible range, as determined from the Type I mixture, would be regarded as a signal that these represent presence of some unused reactant. However, in some cases, these might well belong to a new compound.
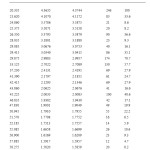 |
Table1: HgCl2 Click here to View table |
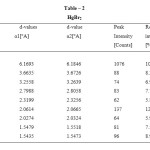 |
Table2: HgBr2 Click here to View table |
With this background in mind we study the data of the mercury halides and their mixtures mentioned above.
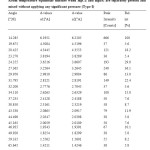 |
Table3: Room temperature equimolar mixture when HgCl2 and HgBr2 are separately pressed and mixed without applying any significant pressure (Type-I) Click here to View table |
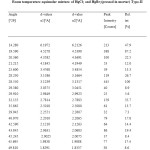 |
Table4: Room temperature equimolar mixture of HgCl2 and HgBr2(pressed in mortar) Type-II Click here to View table |
By comparing the three most prominent peaks of HgCl2 and the single most prominent peak of HgBr2 (all the other peaks of HgBr2 being very insignificant), with the corresponding peaks in the Type I mixture we can find out the maximum permissible deviation in the d-values, of a prominent peak, when the compound be present in a mixture.
By the above comparison, we may ascribe the maximum deviation to the d-values of 2.6263 (a1) in the Type I mixture, if we identify it with the d-value of 2.7022(a1) in HgCl2 with an intensity of 57.7%. This would be about 0.08°A. However, if we identify the peak at d=2.8121 (a1), in the Type-I mixture, with the d value of 2.7022 (a1) in HgCl2, the permissible deviation would be about 0.1°A. To be on the safe side, we take the latter to be the permissible deviation for HgCl2 in a mixture. Any difference in the d-value within 0.1°A from those for the HgCl2 peaks would be taken to signal presence of HgCl2 but d-values outside the maximum upper limit would generally mean absence of HgCl2 and presence of some other compound. We see that for all the other prominent peaks of HgCl2 and HgBr2, the expected deviation to be much less than 0.1°A. However, the deviation of the d-values may be larger if the number of compounds in the mixture is large. We may finally add that d-values should always be seen in conjunction with the relative intensity.
In the above light, we examine our products. The presence of the most prominent peak at d = 3.1239, in the Type II mixture, is a clear indication of the formation of a new product. However, presence of a fairly strong peak at d =6.1972 may be taken as a clear indication that HgBr2 is also present in the Type II mixture along with the new product. By comparing its peak intensity counts of 213 with the corresponding counts of 666 in the Type I mixture, we may conclude that the amount of the HgBr2 along with the new product, is about 16%.
Summary of the Analysis of the X-ray Diffractograms
Occurrence of a new prominent peak which is not found in the diffractogram of either of the reactants, may be taken as a strong evidence for the formation of a new product.
If a prominent peak considered as belonging to a reactant is found to have suffered a displacement which is more than what may be regard as permissible. (say, a maximum value of 0.1° A in the d-values, for our system), that may also be taken as belonging to a new product.
Relative intensity consideration is often helpful in assigning a peak to a reactant.
Density Considerations
If the density of a compound AB2 is denoted by and its molecular weight by , it can be easily seen that the density D of a well-packed equimolar mixture of the compounds AB2 and AC2 is given by
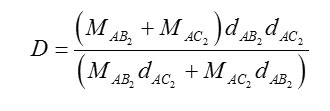
Taking the densities of HgCl2 and HgBr2 obtained from the standard literature as also their molecular weights 12, we get the density D of their equi-molar mixture. The densities of HgCl2 and HgBr2 are given in Table-5, along with the density of HgClBr, as prepared by Method-I. The density regarding HgClBr as an equimolar mixture of HgCl2 and HgBr2 is given inside the bracket. The value of the density for what is called HgClBr is that measured by one of the authors (R.Ahmad). The melting points are taken from ref.12 and are given in Table 5.
Table 5
|
Compound |
Experimental Density (gm/ c.c.) |
Melting point (oC) |
|
HgCl2 HgBr2 HgClBr |
5.44 6.11 5.3 (5.8)* |
276 236 205 |
* The density on the assumption that this is an equimolar mixture of HgCl2 and HgBr2.
Results of the Examination of the Product and the Conclusions
We may examine the products of the reactions using the criteria arrived at above. In this study, we have included only those products for which the X-ray d-values, the FTIR absorption wave numbers as well as the wave numbers of the Raman lines are available. The results are given below (the wave numbers are in cm-1 ).

With the possible exception of the third method for producing HgClBr, in all the other cases, including those for producing HgBrI, a new product which may be regarded as a mixed halide of mercury, is formed, but in all these cases, a small quantity of the constituent di-halides is present along side the mixed halide of mercury. By Method –II of producing HgClBr nano form of HgClBr has been produced. It is clearly seen from all the three studies that the product in each cases is somewhat different. It is intriguing that probably small quantity of one of the reactants remain. This might mean that in a small number of cases a more complicated additive compound is formed. Probably this is arising not due to the instability of the compound formed but due to the formation of a complicated additive compound.
Since all the three kinds of data are not available for the two other slight modifications of Method-I for producing HgClBr, these are not being discussed here. The same applies to the product obtained by the application of high pressure to a pellet of an equimolar mixture of HgCl2 and HgBr2 or HgBr2 and HgI2.
We like to carry out the required analysis that remains in these cases and then to hopefully report these results. However, it seems likely that the mixed halides would have some fraction of the constituent di-halides.
In this light one may have a critical experimental study of the mixed halides of other elements. In fact, one may examine any compounds of the form
etc., in this light one should also have a look at the claims of having produced the single crystals of the mixed halides. Other methods for producing the mixed halides may still be tried out. Use of nano-reactants may make a difference.
Acknowledgement
We are very thankful to Professor M.Z.R. Khan, Retd. Professor of Physics, A.M.U. Aligarh, for several discussions which have proved extremely useful to us. Thanks are also due to Prof. M.A. Wahab, Ex-HOD, Department of Physics, JMI, New Delhi for X-ray measurements. We would also like to thank Prof. Ajay Gupta, Former Centre Director IUC – DAE, CSR, Indore and scientists Dr. Deshpandey and Dr. Vasant Sathe of IUC-DAE, CSR, Indore for FTIR and Raman studies. We would also express our thanks to Dr. Shakir Ali, Dr. Abdullah and Dr. Sidharth for help in X-ray measurements. The helpful attitude of the Ex-Head of the Chemistry Department, JMI Prof. Kishwar Saleem is gratefully acknowledged. We are also thankful to Prof. Sharif Ahmad, Ex-Head Department of Chemistry, JMI, New Delhi.
References
- Mercury and the environment, ‘Basic facts’, Environment Canada, Fedral Government of Canada, (2004), (Retrieved on 2008-3-27).
- Rastogi, R. P.; Dubey, B. L. J. Am. Chem. Soc. 1967, 89, 200.
- Ammlung, R. L.; Brill, T. B. Inorg. Chem. Acta. 1974, 11, 201.
- Griffiths, T. R.; Anderson, R. A. J. Chem Soc. Dalton Trans. 1980, 2, 205.
- Strull, A.; Givan, A.; Loewenschuss, A. J. Mol. Spect. 1976, 62, 283.
- Bloom, H.; Anthony, R. G. Australian J. Chem. 1972, 25, 23.
- Rastogi, R. P.; Dubey, B. L.; Agrawal, N. D. J. Inorg. Nucl. Chem. 1975, 37, 1167.
- Mehdi, S.; Ansari, S. M. J. Solid State Chem. 1981, 40, 122.
- Ahmad, R.; Ali, J. Oriental J. Chem. 2010, 26, 1127.
- Wilson, A. J. C. Proc. Phys. Soc. 1962, 80, 286; 1962, 41, 81
- Warren, B. E. X-ray diffraction, Addison Wesley Publishing Co. London, 18, (1969).
- Lide, D.R. CRC Handbook of Chemistry and Physics, CRC Press, (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









