The Results of Physico - Chemical and Microscopic Investigations of Phosphorus Sludge
U. B. Nazarbek*1, U. Besterekov1, S. P. Nazarbekova2, A. A. Bolysbek1,
1Department of Chemical technology of inorganic substances M.Auezov South Kazakhstan State University, South-Kazakhstan region, Republic of Kazakhstan
2Department of Chemistry, M.Auezov South Kazakhstan State University, South-Kazakhstan region, Republic of Kazakhstan
DOI : http://dx.doi.org/10.13005/ojc/310123
Article Received on :
Article Accepted on :
Article Published : 24 Feb 2015
The results of physico-chemical and microscopic investigations of phosphorus sludge are considered. It was established that phosphorus sludge does not meet regulatory requirements for mineral fertilizing on granulometric composition. On chemical composition, phosphorus sludge is close to original phosphate raw material of Karatau deposit, but it differs from latter by its less content of phosphate component and presence of constituents of charge mixture – quartzite and coke. The results of microscopic investigations show that phosphorus sludge structure contains mainly calcium silicates, diopside, and potassium aluminosilicates. The presence of latter ones allows that phosphorus sludge is a valuable secondary phosphate raw material, quite suitable for recycling of it into phosphorus, potassium containing complex mineral fertilizer.
KEYWORDS:phosphorus sludge; microstructure; chemical analysis; granulometric composition; elemental composition; sieve analysis
Download this article as:| Copy the following to cite this article: Nazarbek U. B, Besterekov U, Nazarbekova S. P, Bolysbek A. A. The Results of Physico - Chemical and Microscopic Investigations of Phosphorus Sludge. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Nazarbek U. B, Besterekov U, Nazarbekova S. P, Bolysbek A. A. The Results of Physico - Chemical and Microscopic Investigations of Phosphorus Sludge. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7398 |
Introduction
At the present time, the dumps of the former Shymkent production organization (ShPO) “Phosphorus” in southern Kazakhstan in six sludge storages with a total area of over 30 hectares contains about 500,000 tons of phosphorus sludge. Recycling of it is important from both an environmental and economic points of view. Phosphorus sludge is formed due to the specificity of modern ore-thermal technology of extraction of elemental phosphorus from phosphorite, which is chemically identical to raw material as phosphorite1,2,3,4. However, it follows that phosphorus sludge differs from primary raw material by its composition, physico-chemical and microscopic properties and characterized by a number of individual properties, as phosphorus sludge is formed from a charge mixture consisting of phosphorite, coke and quartzite. In addition, a granulometric composition of phosphorus sludge will also significantly differ from the composition of a charge mixture mass of phosphorus production.
At this moment, practically all phosphorus sludge of ShPO “Phosphorus” has undergone a special vapor-heat treatment in LP “Kaynar” for the purpose of separation of free phosphorus from its composition, which is approximately composed over 90 thousand tons. Consequently, initial phosphorus sludge has undergone a number of changes, which affect on its characteristic indicators and properties5. In this regard, the study of granulometric composition, physical and chemical properties and peculiarities of sludge secondary phosphate raw materials are the actual problem.
Materials and Methods
Samples of phosphorus sludge uniformly were collected from different levels of sludge tanks. Sieve analysis of samples was carried out according to the State Standard 21560.-82 “Mineral fertilizers. Method for determination of granulometric composition” using a vibrating sieve rumble of “Analysette 3” of PRO model.
Chemical analysis of phosphorus sludge was carried out in compliance with the regulatory requirements of the State Standards: 3760-79, 9264-79, 9336-75, 3765-78, 3773-72, 4109-79, 6709-72, 4198-75, 4461-77, 3652-69, 4209-77, 5850-72, 4234-77, 4204-77, 3118-77, 10484-78, 4212-78, 25336-82, 1770-74, 20292-74, 4144-79, 18300-72, 24363-80, 10652-73, 10398-76, 12083-78, 4478-78, 199-78, 3117-78, 61-75, 25794-83 and others.
Phosphorus sludge was subjected to microstructural analysis using a scanning electron microscope JEOl, brand JSM 6490 LV.
Microstructure and element-weight composition of phosphorus sludge samples were studied at 40 and 500 times magnification.
Results and Discussion
Figure 1 presents the results of sieve analysis of the investigated phosphorus sludge of LP “Kaynar”. The data from Diagram 1 show that 1/3 part of the phosphorus sludge is equal to the share of fine fraction with a particle size of 0,100-0,315 mm. In total weight of phosphorus sludge about 5% is a large fraction with a size of greater than 1.4 mm. The share of dust particles of phosphorus sludge is about 20%. Basic mass of phosphorus sludge constitutes fractions with dimensions: 1.0 -1.4 mm; 0,71-1,0 mm; 0,50-0,71 mm; 0,315-0,500 mm. As it’s seen from the data of diagram 1, mass fraction of these fractions is approximately the same, and the average is 12%.
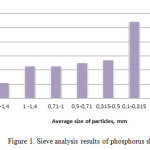 |
Figure1: Sieve analysis results of phosphorus sludge Click here to View figure |
It follows that the phosphorus sludge by natural granulometric composition does not meet modern requirements for products of fertilizing purposes6. In developing the method and recycling technology of it into the fertilizer it is necessary consider these features and provide opportunities for a preliminary enlargement of particles of slurry mass or finish granulation of obtained desired product on the basis of phosphorus sludge.
Results of chemical analysis of phosphorus sludge samples are given in Tables 1,2. Phosphorus sludge samples were taken from all six sludge tanks with different markers in height. From which it is visible that the test samples as a whole on chemical composition is not significantly differed from each other and close to the original phosphate raw of Karatau deposit with inclusions composed of charge mixture mass – quartzite and coke. Comparative analysis of the data of Tables 1,2 with the known data about the chemical composition of phosphorites of Karatau7 shows that the noticeable reduction of concentrations of P2O5, Na2O, CaO, Al2O3, F, and the substantial increase of K2O, SiO2, MgO, C and Σ heavy metals contents are observed in the phosphorus sludge. It follows that the fertilizing value of phosphorus sludge by P2O5 lower than phosphorite and phosphorus sludge is advantageously differed by the content of K2O from the latter one. Moreover, practically three times increase of the content of silicon oxide, two times increase of the content of magnesium oxide and the appearance of free carbon, as well as exposure of noticeable presence of heavy metals in the phosphorus sludge causes the necessity to incorporate these features at the development of production technology of quality fertilizing product on the basis of phosphorus sludge.
Data of the Figure 2 follows that the general structure of the analyzed sample is characterized by inclusions of large minerals of calcium silicates 2CaO · SiO2 and small grains of wollostonite CaO · SiO2. It is also observed the presence of single minerals of CaO · Al2O3, compounds of potassium aluminosilicates, calcium phosphate 3CaO · P2O5 and fluoride CaF2.
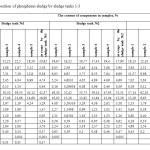 |
Table1: Chemical composition of phosphorus sludge by sludge tanks 1-3 Click here to View table |
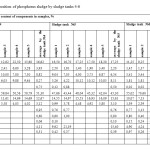 |
Table2: Chemical composition of phosphorus sludge by sludge tanks 4-6 Click here to View table |
Figure 1 (spectrum 2) shows the part of an investigated sample of phosphorus sludge at 500 times magnification. The analyzed part clearly illustrates the presence of minerals 2CaO · SiO2, CaO · Al2O3, CaO · SiO2 and calcium phosphate and fluoride here. The presence of hydrated compounds of calcium phosphate in the studied sludge sample is characterized by film and unformulated structure of minerals.
In the spectrum 3 (Figure 2) is also given the part of the testing sludge sample, obtained at 500 times magnification. Basic mass of this structure is presented by minerals 2CaO · SiO2. Here, the inclusions of metallosilicate and calcium aluminate are also presented. Between silicate and aluminate phases is given insignificant inclusions of ferrites of calcium (CaO · Fe2O3), diopside (CaO · MgO · 2SiO2) and calcium fluoride8. Calcium silicates and aluminates constitute 60-65% in total mass of microstructures of the samples.
The inclusion content of calcium ferrites and diopside do not exceed 13-15%. Weight content of calcium fluoride inclusions reaches up to 5% (Figure 2, Table. 3).
 |
Figure2: Microstructure of the samples, enlarged 40 and 500 times (spectra 2 and 3). Click here to View figure |
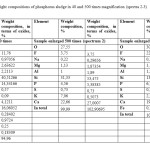 |
Table3: The elemental and weight compositions of phosphorus sludge in 40 and 500 times magnification (spectra 2-3) Click here to View table |
Analysis of the microstructure of investigated samples of phosphorus sludge shows that their structure contains mainly calcium silicates, diopside and potassium aluminosilicates. By this phosphorus sludge is enriched to a certain extent by alkali oxide – K2O. Moreover in phosphorus sludge the ratio of SiO2 \ CaO ≈ 2 that practically corresponds to the module of acidity of initial charge mixture is 0.85. Consequently, in the presence of residual free phosphorus in phosphorus sludge is possible the formation of silicon pyrophosphate that contributes to an increase of the content of available phosphorus5.
Thus, the results of conducted studies suggest that phosphorus sludge is a valuable secondary phosphate raw material, quite suitable for recycling it into phosphorus, potassium, containing complex mineral fertilizer.
References
- Akhmetov, T.G., Chemical technology of inorganic substances, Moscow, Chemistry, 1998
- Pozin, M.Ye., Tehnology of mineral salts and fertilizers, Leningrad, Chemistry, 1986
- Postnikov, N.N., Thermal phosphoric acid, Moscow, Chemistry, 1972
- Yershov, V.A., Pimenov S.D., Electrothermics of phosphorus, St-Petersburg, Chemistry, 1996
- Batkayev, R.I., Development of obtaining technology for marketable products from industrial wastes of phosphorus production: Dissertation thesis of doctor of engineering, 2010
- Mineral fertilizers. Testing Methods: Collection of State Standards: Publishing of standards, 2003
- Kiperman, Yu.A., Phosphates in XX1 century, Almaty-Taraz-Zhanatas, 2006.
- Gorshkov, V.G., V.V. Timashev, V.G. Savelev, Physico-chemical analysis of cohesive materials. High School, 1981

This work is licensed under a Creative Commons Attribution 4.0 International License.









