The Effect of Inorganic and Organic Pre-reducing Agents on Selenium Analysis in Tomato Sample using Microwave-Assisted Digestion Followed by FI-HGAAS
Nunticha Limchoowong1, Pornpimon Tan-Umporn1, Lampaen Laijungred1, Suchila Techawongstein2 and Saksit Chanthai1*
1Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand
2Department of Plant Science and Agricultural Resources, Faculty of Agriculture, Khon Kaen University, Khon Kaen 40002, ThailandDOI : http://dx.doi.org/10.13005/ojc/310118
Article Received on :
Article Accepted on :
Article Published : 21 Feb 2015
Optimum pre-reducing agents used to convert Se(VI) to Se(IV) for speciation analysis of inorganic selenium in tomato were investigated by FI-HGAAS. It was found that optimal conditions of Se(IV) consisted of 2 M HCl as carrier and 0.3%(w/v) NaBH4 in 0.2%(w/v) NaOH as reducing agent. Concerning the completely pre-reduction of Se(VI), various reducing agents were studied including KCl, KBr, KI, ascorbic acid and thiourea in association with dilute and concentrate HCl and effect of temperature. From results, two optimal pre-reducing agents:0.5%(w/v) KI/conc. HCl and 0.5%(w/v) thiourea/conc. HCl and heating for 30 min were chosen, giving a linearity between 5 and 25 ng/mL. LOD and LOQ were 0.5 and 2 ng/L, respectively. Both of pre-reducing agents were then applied to tomato digest based on dry basis of its sample, which comparatively resulted in 5.10 mg Se(IV)/kg and 29.12 mg Se(VI)/kg using 0.5%(w/v) KI/conc. HCl, and 3.84 mg Se(IV)/kg and 22.29 mg Se(VI)/kg using 0.5%(w/v) thiourea/conc. HCl.
KEYWORDS:Selenium; Speciation; Pre-reduction; Tomato; FI-HGAAS
Download this article as:| Copy the following to cite this article: Limchoowong N, Tan-Umporn P, Laijungred L, Techawongstein S, Chanthai S. The Effect of Inorganic and Organic Pre-reducing Agents on Selenium Analysis in Tomato Sample using Microwave-Assisted Digestion Followed by FI-HGAAS. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Limchoowong N, Tan-Umporn P, Laijungred L, Techawongstein S, Chanthai S. The Effect of Inorganic and Organic Pre-reducing Agents on Selenium Analysis in Tomato Sample using Microwave-Assisted Digestion Followed by FI-HGAAS. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7324 |
Introduction
Selenium is an important trace element in biological and environmental systems1. This element is an essential trace mineral in the body. It is important part of antioxidant enzymes that protects cells against the effect of free radicals that are produced during normal oxygen metabolism. Selenium is also essential for normal functioning of the immune system and thyroid gland. Nevertheless, the biochemistry of selenium has shown that it has dual roles and the excess intake of selenium may be harmful to human body 2. For the levels of selenium in toxicology, it has long been known that great differences in toxic properties occur among the various species of an element, because they follow different metabolic pathways. In particular, selenium absorption, retention and distribution within the body, and the amounts, forms and routes of excretion vary with the chemical forms and amounts of the element ingested 3. It can be found naturally in four valence states including selenide, Se(-II), elemental Se(0), selenite, Se(IV) and selenate, Se(VI). Although precise feeding comparisons of the relative toxicity or nutritional activities of the different selenium compounds have not yet been made, it has been reported that the inorganic forms of Se are more toxic than the organic ones 4. For humans and most other mammals, the toxicity of inorganic selenium increases in the order: selenite < selenate < selenide 5. Selenium is primarily present as inorganic forms of Se(IV) and Se(VI) in the majority of environmental matrices such as water and soil 6. The level of selenium species is common in the range of µg/L. Thus, the study of the selenium species in tomato would provide a better understanding of the requirements of this element in the living system.
Most methods for the selenium determination have reported as total selenium 7. However, relatively few analytical methods have been applied for the selective determination of the different selenium species present in various matrices. Chromatographic techniques coupled with different detector systems have been extensively used for the separation and determination of all selenium species. This approach minimizes interferences from the matrix. However, errors may arise from a lack of efficiency in the separation, e.g. due to incomplete retention on the column, decomposition of the species, incomplete recovery of the eluate or peak overlap 8. There are few electrochemical methods that can be applied for selenium speciation in biological fluids and environmental samples 7,9,10. However, the complete mineralization or conversion of Se(VI) to Se(IV) (the electroactive form) makes sample preparation more complex and increases the risk of sample contamination and of losses of selenium. Spectrofluorimetric methods have been also used for the determination of the different inorganic species of selenium7,9. Although very sensitive, an initial acid digestion step is required for this technique and the analytical figures of merit may be markedly influenced by extraction solvent, temperature and the hydrogen ion concentration. The detection of methyl selenide species is carried out after gas chromatographic (GC) separation on the basis of their different retention times with a wide variety of detection methods such as electrothermal atomic absorption spectrometry (ETAAS)11 and microwave-induced helium plasma detection (MWPO)12. The most commonly applied technique for inorganic selenium species determination is based on hydride generation (HG) with atomic absorption spectrometric (AAS) detection9. In all of these techniques, Se(IV) is determined directly after derivatization process, whereas Se(VI) is determined by difference value after reduction, and the organic selenium content is also determined by difference after destroying organic compounds by oxidation. These techniques are highly sensitive and yield detection limits in the ngL-1 and are not subject to major interferences or high background noise levels13. The severe and systematic imprecision reported for this technique are almost due to the use of improper sample decomposition. The tolerance limits for other hydride-forming elements in the determination of selenium could be improved by one or two orders of magnitude by using a flow injection (FI) instead of a batch system and optimizing the analytical conditions systematically 14. According to the literature on total selenium determination in biological and environmental materials, the combination of FI techniques with HGAAS is an accepted method 15-18. In previous study, only one paper has described the FI closed system with thermal heating at 140°C for the determination of Se(IV) and Se(VI) by HGAAS with on-line pre-reduction of Se(VI) to Se(IV)19. No losses of selenium occur and the method has been successfully applied to the speciation of both inorganic species.
Although, FI-HGAAS can be used to determine Se(IV) only from the mixture of Se(IV) and Se(VI) solution, the concentration of Se(VI) is calculated by the difference between total inorganic Se and Se(IV). In the present study,the prevention of the back-oxidation of Se(IV) to Se(VI) by using various pre-reducing agents is aimed. Only Se(IV) forms the hydride, and so Se(VI) must be pre-reduced to Se(IV) if total selenium is to be determined. When NaBH4 is used as a reducing media for the determination of selenium by HGAAS, only SeH2, the Se(IV) redox state is determined directly. Total inorganic Se is determined after quantitative reduction of selenate to selenite. Considering that FI-HGAAS cannot detect Se(VI) directly, Se(VI) has to be reduced to Se(IV) first. Thus, the pre-reducing step to convert Se(VI) to Se(IV) is still necessary.
In this study, the optimal pre-reducing agents to convert Se(VI) to Se(IV) for ultra-trace analysis of an inorganic Se species and total Se are investigated using microwave-assisted digestion (MAD) and determination by FI-HGAAS. Application of the optimal pre-reducing agent of the Se species is then carried out with five varieties of tomato samples.
Materials and Methods
Chemicals and Reagents
All aqueous solution were prepared with deionized water (Milli Q Millipore 18.2 MΩ cm of resistivity) by Simplicity water purification system, Model Simplicity 185, Millipore Corporation (USA). All glass wares were cleaned by soaking in dilute 1%(v/v) HNO3 overnight and rinsed two times with deionized water prior to use. A 1,000 mg/mL Se(IV) stock solution was prepared from SeO2 (Carlo Erba, France) in HCl (Carlo Erba, France). A 1,000 mg/mL Se(VI) stock solution was prepared from Na2SeO4 (Sigma Aldrich, USA) stored in a refrigerator at 4°C. Lower concentrations were prepared on the day by diluting the stock solution with 0.1 mol/L HCl. The 0.3% (w/v) of NaBH4 was prepared daily by dissolving 0.75 g of NaBH4 (Lab Chem, France) in 0.2% (m/v) NaOH (Carlo Erba, France) and diluting quantitatively to 250 mL with same solution. The carrier solution was 2.0 mol/L HCl.
The pre-reducing agents included only conc. HCl or the solution containing KBr (Asia Pacific specialty, Australia) or thiourea (BDH laboratory supplies, England) in the presence of either conc. HCl or dilute HCl were employed to reduce Se(VI) to Se(IV). For digestion of tomato sample, 65% (w/v) HNO3 (Lab Scan Asia, Thailand) was used.
Instruments
Selenium measurements were made using an atomic absorption spectrometer of the Perkin-Elmer AAnalyst 100 (Connecticut, USA) equipped with a flow injection analysis system Model FIAS-100 (Perkin Elmer Instruments, USA), used for continuous flow hydride generation. The FIAS-100 flow injection system consists of one peristaltic pump, five-port valve and a regulated gas control. Argon gas was used as carrier gas for the transposition of selenide (SeH2) from the gas-liquid separator to quartz tube atomizer. The selenium hollow cathode lamp (Victoria, Australia) was set at 196.0 nm wavelength, 16 mA lamp current and 2.00 nm slit width. A flow injection hydride generation system with a heated quartz tube atomizer was used for hydride generation and coupled to the AAS. PTFE tubing was used to transfer sample and solutions. The atomic absorption signal was measured as a peak height mode providing an analytical curve. The peristaltic pump, injection time and data acquisition were controlled through Perkin Elmer AA Winlab atomic absorption software version 3.2. The analytical conditions for FI-HGAAS determination are shown in Table 1.
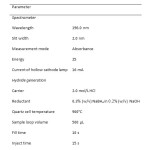 |
Table1: The operational conditions for the determination of Se by FI-HGAAS |
Materials
Tomato samplewassoaked and washed with tap water followed by deionized water. The Seeda tomato as a model as a model sample was homogenized. The homogenized fresh matter was transferred into PTFE centrifuge tube and freeze-dried (SCANVAC centrifuge for vacuum concentrator freeze-dry) at -20°C. Once the samples were powdered and dried. They were kept at -20°C prior to digestion. Approximately 0.2-0.5 g of the sample were weighed accurately, added 7 mL 65% (w/v) HNO3 and 2 mL 37% (w/v) HCl. The sample mixture was digested under high temperature and pressure conditions using a microwave(Anton Parr 3000, Austria)unit at 220°C, 32.9 psi, 800 W for 45 min. After cooling, each solution was made up to 25 mL final volume with 0.1 mol/L HCl. The digested clear sample solutions were further treated for the pre-reduction step of Se(VI) to Se(IV) prior to measurement by FI-HGAAS as described below.
Pre-Reduction of Se(VI) To Se(IV)
Concerning the pre-reduction of Se(VI), various reducing agents were commonly used including some potassium halide salts (KCl, KBr and KI) and some organic compounds such as ascorbic acid and thiourea. The concentration of the pre-reduction agent was investigated in the ranges of 0.5, 1.0, 2.0, 4.0, 6.0, 8.0, 10.0 and 12.0 % (w/v). The experimental parameters mainly affecting on the pre-reduction step were as follows.
Effect of Pre-Reducing Agent
To a standard solution of 20 µg/L Se(VI), 1 mL each of the pre-reducing agent was added, diluted to 10 mL with water and analyzed by FI-HGAAS.
Effect of Pre-Reducing agent in Association with Dilute HCl or conc. HCl
1. Effect of the pre-reducing agent in association with dilute HCl: To standard solution of 20 µg/L Se(VI), the pre-reducing agent and 2 mL of 6 mol/L HCl were added, diluted to 10 mL with deionized water and analyzed by FI-HGAAS.
2. Effect of the pre-reducing agent in association with concentrate HCl: To standard solution of 20 µg/L Se(VI), the pre-reducing agent and 2 mL of conc. HCl were added, diluted to 10 mL with deionized water and analyzed by FI-HGAAS.
Effect of Temperature
- Effect of the pre-reducing agent and heating: To standard solution of 20 µg/L Se(VI), the pre-reducing agent was added 1 mL, heated in a water bath at 70-80°C for 30 min, cooled down, diluted to 10 mL with deionized water, and analyzed by FI-HGAAS.
- Effect of the pre-reducing agent in association with dilute HCl and heating: To standard solution of 20 µg/L Se(VI), the pre-reducing agent 1 mL and 2 mL of 6 mol/L HCl were added, heated in a water bath at 70-80°C for 30 min, cooled down, diluted to 10 mL with deionized water, and analyzed by FI-HGAAS.
- Effect of the pre-reducing agent in association with conc. HCl and heating: To standard solution of 20 µg/L Se(VI), the pre-reducing agent 1 mL and 2 mL of conc. HCl were added, heated in a water bath at 70-80°C for 30 min, cooled down, diluted to 10 mL with deionized water, and analyzed by FI-HGAAS.
Results and Discussion
The Optimal Pre-Reduction Agents
Without HCl, no signal was detected by FI-HGAAS. From the results, five optimal pre-reducing agents were chosen for further studies (Fig. 1) consisting of 0.5% (w/v) KI/conc. HCl/heat, 0.5% (w/v) Thiourea/cocn. HCl/heat, 1.4% (w/v) KBr/dilute HCl/heat, 0.8% (w/v) KBr/conc. HCl/heat, and 1.3% (w/v) KCl/conc. HCl/heat.
 |
Figure1: The effect of various pre-reducing agents for Se(VI) solution on the absorbance of Se(IV) under hydride generation Click here to View figure |
In the cases of 1.4% (w/v) KBr/dilute HCl, 0.8% (w/v) KBr/conc. HCl and 1.3% (w/v) KCl/conc. HCl followed by heating for 30 min, the pre-reducing agents should not be suitable for reducing Se(VI) to be Se(IV) because they were affected due to strong sample matrix.Only two optimum pre-reducing agents: 0.5% (w/v) KI/conc. HCl and 0.5% (w/v) thiourea/conc. HCl and heating for 30 min were chosen for the standard solution of Se(VI). Application of these pre-reducing agents was then carried out with real sample of tomato prepared by microwave-assisted digestion. Consequently, both of them can be used as the calibration curve for the determination of inorganic Se species and total Se in tomato sample as shown in Table 2.
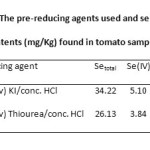 |
Table2: The pre-reducing agents used and selenium contents (mg/Kg) found in tomato sample Click here to View table |
Analytical Figures of Merit
The calibration curve was constructed between 0.5 µg/L and 50.0 µg/L and the linearity was maintained up to 100.0 µg/L with a correlation coefficient (r2) greater than 0.999. Limits of detection (LOD) and quantification (LOQ) calculated as three and ten times standard deviation of absorbance signal of 10 reagent blanks divided by the standard calibration slope of Se(IV) were found to be 0.5 µg/L and 2 µg/L, respectively. This LOD was less than those previously reported 20-21. The precision was less than 10% RSD (n = 3). The precisions of the calibration curve of Se(IV) standard solution between 5 µg/L and 25 µg/L (n = 10) were shown within the acceptable ranges as RSD of 9.4 % and 10.3 % for an inter-day and an intra-day analysis. The results gave rather higher precision for each of the calibration curve for Se speciation analysis.
The speciation study of Se(IV), Se(VI) and both Se species using the selected four pre-reduction systems were demonstrated that all the reducing systems gave rather high content for these Se species (3.84-5.10, 22.29-29-12 and 26.13-34.22 mg/Kg for Se(IV), Se(VI) and total Se, respectively) spiked in the Seeda tomato sample. For sample digestion, the main problem may arise from the peculiar chemical resistance of some organoselenium compounds (selenoamino acid, selenonium salts) to be transformed into inorganic Se salts, selenite or selenate forms. Tomatoes have rather high organic selenium 17. The residues of organic matter, however, could interfere the hydride generation, if the acid digestion of the sample was not completed. Concerning on the inorganic Se species in the tomato samples, the hexavalent Se is found in higher contents than the tetravalent one.
Conclusion
To find out the optimized conditions for Se speciation by FI-HGAAS, using of 2 mol/L HCl solution as carrier associated with a solution of 0.3% (w/v) NaBH4 in 0.2% (w/v) NaOH was run. However, the difference in the absorbance signals obtained from tratavalent and hexavalent selenium depends very strongly on the pre-reduction system used under the experimental conditions. Since Se(VI) gives very low signal in HGAAS system, the off-line pre-reduction of Se(VI) to Se(IV) must be completed. For this reason, the pre-reduction procedure for the digested samples was also optimized once in details. The sample digestion was conducted with a microwave unit under high temperature and pressure. It is fast and safe compared with common acid digestion in hood. Consequently, the proposed method was applied for analysis of inorganic Se in tomato. The pre-reduction step made possibly the completeness of the inorganic Se species determination. The use of a mixture of the pre-reducing agents permitted the quantitative reduction of Se(VI) to Se(IV) under mild experimental conditions.
Acknowledgements
This research was supported by the Higher Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster of Khon Kaen University. The Center of Excellence for Innovation in Chemistry (PERCH-CIC) and Commission on Higher Education, Ministry of Education.
References
- Rayman, M.P.; Phil, D. Lancet.2000, 356, 233–241.
- Sun, Y.C.; Chang, Y.C.; Su, C.K. Anal Chem.2006,78, 2640–2645.
- Underwood, E.I., Selenium in Trace Elements in Human and Animal Nutrition. New York,(1977).
- Narasaki, H.; Mayumi, K. Anal Sci.2000, 16, 65–68.
- Petropoulou, M.O.;Michalke, B.;Kavouras, D.;Schramel, P.Anal ChimActa.2003, 478, 219–227.
- Campillo, N.;Aguinaga, N.;Viñas, P.;López-García, I.; Hernández-Córdoba, M.J. Chromatogr A.2005, 1095, 138–144.
- Alaejos, M.; Romero, C.Chem Rev.1995, 95, 227-257.
- Raptis, S.E.; Kaiser, G.;Tolg, G. Anal Chem.1983, 316, 105-123.
- Mufioz, R.;Donard, O.;Camara, C.;Ouevauviller, P.AnalyChimActa.1994, 286, 357-370.
- Bryce, D.W.;Izquierdo, A.;Luque de Castro, M.D. Anal ChimActa.1995, 308, 96-101.
- Jiang, S.;Robberecht, H.; Adams, F.; Van der Berghe, D. ToxicolEnviron Chem.1983, 6, 191-201.
- Olsen, K.B.;Sklarew, D.S.; Evans, J.C.SpectrochimActa.1985, 40, 357-365.
- Verlinden, M.;Deelstra, H.A.;Adrianensses, E. Talanta,1981, 28, 637-646.
- Welz, B.;Stauss, P.SpectrochimActa B.1993, 48, 951-976.
- Chan, C.C.Anal Chem.1985, 57, 1482-1485.
- Fang, Z.; Xu, S.; Wang, X.; Zhang, S.Anal ChimActa.1986, 179, 169-179.
- Ikeda, M.Anal ChimActa.1985, 170, 217-224.
- Pyen, G.S.; Browner, R.F. ApplSpectro.1988, 42, 508-512.
- Cobo, M.G.; Palacios, M.A. Anal ChimActa.1993, 283, 386-392.
- Li, F.;Goessler, W.;Irgolic, K. Anal Com. 1998, 35, 361-364.
- Sigrist, M.; Brusa, L. Campagnoli D, Beldoménico H.Food Chem.2012,134, 1932-1937.

This work is licensed under a Creative Commons Attribution 4.0 International License.









