Systhesis and Characterization of Some Chromium (Iii) Schiff Base Complexes
Kalpana Raikwar1* and D. D. Agarwal2
1Deptt. of Chemistry, Govt. P.G. College, Guna 473001(M.P.) 2SOS, Deptt. of Chemistry, Jiwaji University, Gwalior 474011(M.P.)
DOI : http://dx.doi.org/10.13005/ojc/310168
Article Received on :
Article Accepted on :
Article Published : 14 Feb 2015
Chromium (III) Schiff base complexes have been synthesized using Schiff base formed by the condensing by the respective aldehyde with diamene in ethanol (rectified). All the complexes were characterized on the basis of their micro analytical data, elemental analysis, melting points, IR, UV- vis spectra and magnetic moment properties. The magnetic moment suggest that all the complexes are in the range 3.6-4.1 indicating the three unpaired electron. The infrared spectrum of the complexes under investigation confirmed the site of chelation, hence the complexes showed stretching due to n(C=N) and n(C-O) which suggest the involvement of phenolic group in the coordination. The UV-Vis spectral data shows the π →π* transition which suggest to involves molecular orbital π localized at the azomethane linkage.
KEYWORDS:Schiff bases; Salicylaldehyde; Characterization; IR; Magnetic moment; Dimethyl formamide
Download this article as:| Copy the following to cite this article: Raikwar K, Agarwal D. D. Systhesis and Characterization of Some Chromium (Iii) Schiff Base Complexes. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Raikwar K, Agarwal D. D. Systhesis and Characterization of Some Chromium (Iii) Schiff Base Complexes. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7237 |
Introduction
Schiff base complexes find important position in coordination chemistry and schiff base complexes with transition as well with heavy metals are used in organic synthesis, analytical reagents, and catalysts and in medicine1. Certain oxo metal complexes of U2,3, Os4, 5,W6 and Cr7,8 play an important role in organic synthesis because of their ability to selectively attach oxygen atom to olefins and other organic substrates. Recently metal catalyst of various organic oxidations effectively promoted chromium complexes acting via. their oxidation states, presumably chromium(III) has been reported9-13. Schiff base ligands14 are able to coordinate with metal through imine nitrogen and another group usually linked to the aldehyde. Infect Schiff bases are able to stabilize many different metals in various oxidation states, controlling the performance of metal and a large variety of useful catalytic transformations15,16. Chromium(III) complexes containing schiff bases and substituted benzimidazole / benzoxazole have attracted many workers17-19 due to their biological, pharmacological, clinical and analytical importance. The biologically important form of chromium is the trivalent ion, Cr3+, which is required for carbohydrate and lipid metabolism in mammals20.
The coordination chemistry of Schiff bases derived from the reaction of salicylaldehyde and o-diamines has been the subject of many studies and a number of them are used as oxygen carriers to mimic complicated biological systems21, 22. They are also efficient reagents in trace analysis of some metal cations23. Most of the Schiff bases are chemically unstable and show tendency to be involved in various equilibrium, like tautomeric inter conversions, hydrolysis or formation of ionized species24.
Experimental
The material and reagents used in this study were laboratory pure chemical. The chemical components needed for the synthesis of desired schiff base complexes were chromium trioxide, thionyl chloride, salicylaldehyde, 1,3-diamino ethaane, ethylene diamine, p-cresol, 2-isopropyl phenol, 2-tert, butyl phenol, glycerol, boric acid, hexamine, ethanol, dichloro methane chloroform, methanol, dichloromethane, dimethyl formamide (DMF).
Synthesis of Schiff base Ligands
All the Schiff base ligands viz; bis(salicylaldehyde)1,2- diaminoethane(H2SALEN), bis (2-hydroxy-5-methyl benzaldehyde) 1,2- diaminoethane(H2HMBEN), bis(2-hydroxy-3.Isopropyl benzaldehyde) 1,2-diaminoethane(H2HPBEN), bis(3-tert,butyl-2-hydroxy benzaldehyde) 1,2-diaminoethane(H2BHBEN),were synthesized by the procedure as given below.
All the Schiff bases were synthesized by condensing the respective aldehyde with diamine (in 2:1 ratio) in ethanol (rectified). The mixture was refluxed for 3 hours on cooling crystals were separated out, which were filtered washed with water and recrystallized from ethanol. The characteristics of all synthesized Schiff bases are given in table-1.
Synthesis of Cr(III)Schiff Base Complexes
Schiff base complexes were synthesized by taking chromium tri chloride + ligand (SALEN) + sodium carbonate 1:1:3/4 molar ratio using solvent system ethylene glycol + water + methanol in (1:1:3 w/w ratio) and the contents were refluxed for about 5 hours and color was checked. After the completion of reaction the contents were concentrated which results in a solid which was filtered off and dissolved in sodium hydroxide solution, yellow needle like crystals of [Cr (salen) (H2O)2(Cl)] was obtained after nutralization using dil. hydrochloric acid. Similar procedure was adopted for the preparation of other Chromium(III) Schiff bases complexes.
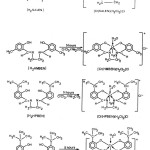 |
Figure Click here to View figure |
Measurements
The Schiff base ligand and its complexes under investigation were subjected to (C, H and N) elemental analysis which performed at analytic unit of the D.R.D.E. Gwalior using CARLO-ERBA and Haereus C, H, N, O–rapid elemental analyser. Melting points were measured in “TEMPO” electrically operated melting point apparatus. For conductance measurement solutions (10-3M) of metal complexes were prepared in dimethyl formamide. The conductivity of the solution was measured using a digital conductivity meter.
The magnetic moment measurements were carried out at room temperature using a Gouy balance on a Brucker magnet B-E15. The electronic spectra of complexes has been recorded in dimethyl formamide using SHIMADZU UV- 160 A UV visible spectrophotometer in the range 200-800 nm.
Results and Discussion
Elemental Analysis
The complexes were subjected to chemical analysis and results fall within the range expected for the proposed structures. It is seen that experimentally observed analytical values for C, H and N are in close agreement with the values calculated for the molecular formula assigned to these complexes.
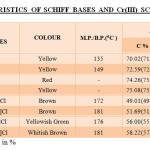 |
Table1: Characteristics Of Schiff Bases And Cr(Iii) Schiff Base Complexes
|
Molar Conductance
The molar conductance of these metal complexes in dimethyl formamide(10-3M) is listed in Table-II. The molar conductivity data for Cr(III) shows that these complexes behave as 1:1 electrolyte. This behavior for these complexes was expected on the basis of molecular formula assigned to these complexes.
I. R. Spectral Studies
The IR spectra in the range of 4000-400 cm-1 for some of the representative complexes were recorded. The characteristic bands are given in TABLE-II. The IR spectrum of Chromium(III) complex shows no strong band in region 3500-3200 cm-1 indicating deprotonation of OH group and its coordination.
In the IR spectrum of [Cr(SALEN)(H2O)2]Cl complex shows bands in the region 3200 -3010 cm-1 which can be assigned to deprotonation of OH group and its coordination with metal.
The IR spectrum of [Cr(HPBEN)(H2O)2]Cl shows that bands in the region 3520-3000 cm-1 are absent which are observed in the IR spectrum of The band due to nC=N is shifted to 1630 cm-1 and overlapped with nC=C. A band at 1350 cm-1 to nC-O stretching which suggest the involvement of phenolic group in the coordination.
The IR spectra of [Cr(HMBEN)(H2O)2]Cl complex shows band in the region 3080-2900 cm-1 which can be assigned to nC-H stretching. The spectra of the ligand showed bands at 1640 and 1610 cm-1 which can be assigned to nC=N and nC=C stretching respectively. The nC=N stretching on coordination was found to shift to lower value on coordination (1630cm-1) and over lapped with nC=C of phenyl ring. The nC-O stretching was found to shift to 1320 cm-1 suggesting its involvement in coordination. The absorption band at 762cm-1 can be assigned to the aromatic vicinal trisubstitution.
ligand suggesting deprotonation of nOH groups and its participation in coordination. Strong peaks in the region 3085-2800 cm-1 can be assigned for nC-H stretching vibration. The peak at 1615 cm-1 can be assigned to nC=N suggesting its participation in coordination. The absorption band at 1320 cm-1 can be attributed to for nC-O stretching mode.
The [Cr(BHBEN)(H2O)2]Cl complex shows absorption bands in the region 3140-2820 cm-1, which can be assigned to nC-H asymmetric stretching vibration of -CH3 and -CH2 groups. Strong bands at 1610cm-1 can be assigned to nC=N suggesting its participation in coordination. The IR spectra also shows strong peaks at 1410 cm-1 and 1460 cm-1 respectively which are due to nC-H stretching of tert.butyl group. The peak at 1320 cm-1 can be attributed to nC-O group.
Electronic Spectra
The UV-visible spectra of some representative Schiff base complexes of Cr(III) were recorded in solvent (dimethyl formamide). The electronic spectra show absorption in the range 550-275nm which can be assigned to n – π* and π- π* transitions. The π- π* transition are suggested to involves molecular orbital π localized at the azomethane linkage. The information regarding the geometry of the complexes could not be ascertained with the observed data.
Magnetic Moment
The magnetic moment for the Cr(III) Schiff base complexes is the range 3.6- 4.1 BM indicating the three unpaired electron.
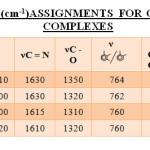 |
Table2: I. R. Frequency(Cm-1)Assignments For Chromium(Iii) Schiff Base Complexes Click here to View table |
References
- Dubey, R.K.; Dubey, U.K.; Mishra, C.M. Ind.J.Chem. 2008, 47A, 1208.
- Thomas, R;, Thomas, K.J.; Parmeshwaram, G. J.Ind.Chem.Soc. 1996, 73, 529.
- Baleizao, C.; Garcia, H. Chem.Rev. 2006, 106, 9 and 3987.
- Bailey, A.J.; Griffith, W.P.; Savage, P.D. J.Chem.Soc.Dalton,Trans. 1995, 21, 3537.
- Ahmad, A.A.; Bengukki, S.A.; Ahshed, O.M. Russian.J.Chem. 2009, 2, 4, 781.
- Ballistereri, F.P.; Tomaselli, G.A.; Toscana, R.M. Spec.Publ.R.Soc.Chem.1995, 148.
- Lorber, C.Y.; Paul, I.; Osborn, J.A. Bull.Soc.Chem.Fr. 1996, 133, 755.
- Dickman, M.H.; Pope, M.T. Chem.Rev. 1994, 94, 569.
- Jin, R.; Cho, C.S.; Jiang, L.H.; Shim, S.C. Bull.Korean.Chem.Soc. 1996, 17, 657.
- Bruice, T.C.; Ostovic, D. J.Am.Chem.Soc. 1998, 110, 6906.
- Khenkin, A.M.; Hill, C.L. J.Am.Chem. Soc. 1993,115, 8178.
- Fillmore, F. Org. Synth.Oxi.Met.Compd. 1986, 41.
- Tretyakov, V.P.; Zimtseva, G.P.; Min’ko. L.A.; Rudakov, E.S. U.Khim.Zh.1990, 56, 51.
- Schiff, H. Ann.Suppl. 1964, 3, 343.
- Gennari. C.; Piarulli, U. Chem.Rev. 2003, 103, 3071.
- Cozzi, P.G. Chem.Soc.Rev. 2004, 33, 410.
- Marie, F.R.; Hans, J.S.; Garrigh. Mc.; Dalton, M., Cornac, T.; Daly. A.; Gilheany, M.C.; Dalton, G. J.Mol.Catal.A.Chem. 2005, 231, 205.
- Shrivastava, H.; Devraj, Y.; Nain, B.U.; J.Inorg.Bio.Chem.2004, 387, 98.
- Cenicern, G.; Agueda, E.; Porlilla, P.R.; Del, F.; Organ, H.; Blum, C. Siliusa. Inorg. Chin. Acta.2002, 331, 59.
- Can-Cheng, G.; He-Ping, Li.; Xiao-Bing Z.; Chin.J.Chem. 2005, 23, 4, 431.
- Hamuryudan, E.; Bekaroglu, A.Z. O.Monatsh.Chem. 2000, 131, 175.
- Karaoglan, G.K.; Actiata, U.; Gill, A. Ind.J. Chem. 2007, 46A, 1273.
- Cimerman, Z.; Galic, N.; Bosner, B. anal.Chim.Acta. 1997, 343, 145.
- Ibrahim, M.N.; Sharif, S.E.A. E-J.Chem. 2007, 4, 531.

This work is licensed under a Creative Commons Attribution 4.0 International License.









