Synthesis of novel cobalt doped zinc oxide/carbon nano composite for the photocatalytic degradation of acid blue 113
S. Sunitha, A. Nageswara Rao*, J. Karthikeyan
Department of Chemistry, Sathyabama University, India
DOI : http://dx.doi.org/10.13005/ojc/310111
Article Received on :
Article Accepted on :
Article Published : 23 Mar 2015
Cobalt doped Zinc Oxide/Carbon nano composite was synthesized by solution combustion method and characterized by X-ray diffractometer, field emission scanning electron microscope and energy dispersive X-ray spectroscopy analysis. This composite shows X-ray diffraction pattern that matched with nano particle of ZnO with wurtzite structure and average grain size was found to be 10.53 nm. . Further the presence of the elements like C, Co, Zn and O was confirmed by energy dispersive X-ray spectroscopy analysis. The effect of Co doping on the photocatalytic activity was investigated by photo degradation of the dye, acid blue 113. This nano composite exhibited better photocatalytic activity when compared to nano ZnO and nano ZnO/C composites.
KEYWORDS:combustion; nano composite; zinc oxide; carbon; cobalt
Download this article as:| Copy the following to cite this article: Sunitha S, Rao A. N, Karthikeyan J. Synthesis of novel cobalt doped zinc oxide/carbon nano composite for the photocatalytic degradation of acid blue 113. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Sunitha S, Rao A. N, Karthikeyan J. Synthesis of novel cobalt doped zinc oxide/carbon nano composite for the photocatalytic degradation of acid blue 113. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=8040 |
Introduction
Water scarcity and its contamination with organic pollutants cause a host of problems both to the environment and community. The scarcity of water has become a serious concern, with the rapid escalation of industrialization towards a developed society. The waste generated from textile, chemical, mining and metallurgical industries is mainly responsible for contamination of water [1, 2]. Semiconductor heterogeneous photocatalysis is a versatile, cost effective and eco-friendly treatment technique for the destruction of organic pollutants in water. Some metal oxide semiconductors such as Fe2O3, ZnO, TiO2, WO3 ,Cu2O, SnO2 are being used as photo catalysts [3-7]. Nowadays nano materials are of great interest for research point of view because the properties of the materials change drastically when the particle size reaches to nanometer range. As the size of the material becomes smaller, the band gap becomes larger thereby changing the optical and electrical properties of the material and making the material suitable for newer applications and devices.
Further the use of nanoparticles exhibit higher photo catalytic activity than their bulk counterparts by increasing their surface area and porosity [8-10]. The most commonly used semiconducting metal oxides are TiO2 and ZnO due to their catalytic activity and stability. However, widespread use of TiO2 is not economical for large scale water treatment operations. Ample information has been documented in literature with regard to higher photocatalytic efficiency of ZnO over TiO2. Moreover, Zinc oxide (ZnO) is an excellent n-type semi conductor with a wide band gap of 3.37 eV and a large excitation binding energy of 60 me V [11,12]. This has attracted the attention of many researchers for the potential use of ZnO in various photo catalytic reactions [13-16]. Nowadays, many methods are in practice to improve the ZnO photostability. This includes surface organic coating of ZnO [17] and surface hybridization of ZnO with carbon and fullerenes C60 [18,19]. In this connection various experiments have been conducted to enhance the rate of photo degradation of ZnO by coupling with adsorbent like activated carbon which provides high concentration of target substances around the catalyst particulate [20,21].
In earlier studies, impurity doping in semiconductor nanoparticles was investigated mainly to enhance the photocatalytic activity, particularly in the UV light region [22]. A number of investigations have focussed on transition metal doped ZnO semi conductor because of its band gap of 3.37 ev, and its large excitation binding energy(60 mev) [23,24] . In the present investigation an attempt was made to synthesize cobalt doped nano zinc oxide/carbon (ZnO/C) composite. Cobalt was chosen for its expected ease in doping due to the similar ionic radius (0.058 nm) to that of Zn (0.060 nm). The synthesized zinc oxide/coarbon/cobalt (ZnO/C/Co) nano composite was characterised by various analytical techniques like XRD, FESEM and EDAX. Its photocatalytic activity is compared with nano ZnO particles and nano ZnO/C composite in the degradation of the dye acid blue 113 (AB 113) in water under UV irradiation in the batch reactor.
Materials and Methods
Materials
The dye, AB 113 commercially known as navy blue S5R with CI number 26360 was obtained from local dye suppliers and the dye was used without further purification. The structure of the dye is shown in figure 1. The dye shows an absorption maximum at 565.5 nm. The solutions of desired concentrations were prepared using double distilled water. The other chemicals Zn(NO3)2.6H2O, Co(NO3)2.6H2O and dextrose were of AR grade obtained from Merck. The pH of the solutions was adjusted between 6.0 and 9.0 using dilute solutions of HCl or NaOH.
Synthesis of ZnO/C/Co Nano Composites
ZnO/C/Co nano composite was synthesized by self propagating solution combustion method [25]. In a typical procedure, 8g of Zinc nitrate and 2g of Cobalt nitrate were dissolved in 20 ml of double distilled water and 3.6 g of dextrose was dissolved in 20 ml of double distilled water in separate beakers. Dextrose solution was heated on a hot plate for 5 minutes, when the solution started boiling, the mixture of zinc and cobalt nitrate solution was added drop wise with constant stirring. The solution on dehydration formed a gel like mass. The beaker was heated in a preheated muffle furnace kept at 4000C. The solution boiled, got ignited and the entire reaction was completed in about 7 minutes. The powder was highly amorphous and the presence of ZnO, Co and carbon was confirmed by EDAX analysis.
Photocatalytic reaction procedure
The photocatalytic degradation of the dye was carried out in a batch reactor of 500 mL capacity, double walled reaction vessel made of borosilicate glass, having dimensions of 16 cm x 5 cm, mounted suitably on a magnetic stirrer. The photocatalytic experiments were performed under identical conditions by using a low pressure mercury vapour lamp (6W, 18 cm along) emitting UV radiation at a peak wavelength of 254 nm. Constant stirring of the solution was ensured with the use of a magnetic stirrer. The temperature of the reaction mixture was maintained constant throughout the reaction time. A batch volume of 250 mL of the dye solution was kept at a distance of 7 cm from the UV source with constant stirring of 60 rpm. In all the experiments a known weight of catalyst was added to known concentration of the dye solution and was allowed for 30 minutes in dark to attain the adsorption equilibrium and then the mixture was irradiated with UV light. A sample (3 mL) was withdrawn at regular intervals and centrifuged. Absorbance of the supernatant solution was measured using UV-visible spectrophotometer at λmax 565.5 nm and returned to the reactor. The structure of the dye, AB 113 is given in figure 1.
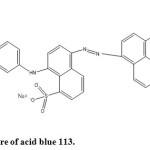 |
Figure1: Structure of acid blue 113. Click here to View figure |
Characterisation techniques
The x-ray powder diffraction patterns (XRD) were obtained using Rigaku X-ray diffractometer, Japan. The crystalline phase of ZnO was identified by comparing with JCPDS card no. 36-1451.The field emission scanning electron microscope (FESEM) and energy dispersive x-ray diffraction (EDAX) photographs of ZnO/C/Co were obtained by using a high resolution scanning electron microscope (Carlzeiss, Supra 55, Germany). UV studies were carried out using SL -210 UV-visible double beam spectrophotometer.
Results and Discussion
Characterisation of the catalyst
The crystal structure of the sample was investigated using XRD (Table 1).
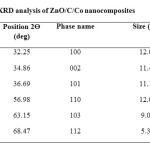 |
Table1: XRD analysis of ZnO/C/Co nanocomposites Click here to View table |
As shown in the figure 2, the ZnO/C/Co nanocomposite matches with the standard diffraction data for the hexagonal ZnO wurtzite structure (JCPDS No. 36-1451).
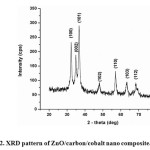 |
Figure2: XRD pattern of ZnO/carbon/cobalt nano composite. Click here to View figure |
No characteristic peaks corresponding to a cobalt oxide phase were observed. This indicates that the concentration of cobalt oxide formed in the sample is too low to be measured [26]. The peaks at 2θ = 31.73°, 34.39°, 36.22°, 47.52°, 56.55°, 62.79°, 66.35°, 67.90°, 69.03°, and 72.54° were assigned to (100), (002), (101), (102), (110), (103) , (112), (004) and (202) planes of ZnO nanoparticles, indicating that the formed nano particle was found to be polycrystalline Wurtzite structure. XRD patterns were identical to the hexagonal phase with Wurtzite structure with space group [C6V = P63 mc) and unit cell parameters a = b = 3.248 Å and c = 5.1 Å. The crystallite size was calculated from the following Debye Scherer formula,
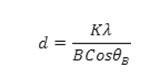
where K is 0.89 (Scherer’s constant), λ is the wavelength of X-rays, θB is the Bragg diffraction angle and B is the full width at half-maximum (FWHM) of the highly intense diffraction peak. Using the Debye-Scherer formula, the average crystallite size of ZnO/C/Co was found to be 10.53 nm respectively. Furthermore, it could be seen that the diffraction peaks shown in figure 2 were more intensive and narrower, implying the good crystalline nature of the ZnO/C/Co nano composite as was observed in ZnO product. The particle size and surface morphology of ZnO/C/Co nanocomposites analysed by FESEM is shown in figure 3.
 |
Figure3: FESEM image of ZnO/carbon/cobalt nano composite. Click here to View figure |
The FESEM image reveals that the entire product is comprised of spherical nano particles with the average size of 22 nm. The EDX studies of ZnO/C/Co nanocomposite confirm the presence of zinc, cobalt and oxygen as the primary components which are shown by figure 4.
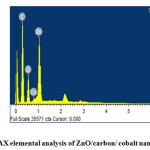 |
Figure4: EDAX elemental analysis of ZnO/carbon/ cobalt nano composite. Click here to View figure |
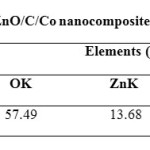 |
Table2: EDAX analysis of ZnO/C/Co nanocomposites Click here to View table |
Photodegradation of Acid Blue 113
The photo degradability of the dye was investigated by exposing the dye solution to UV light in the presence of photocatalysts such as nano ZnO , nano ZnO/C composite and nano ZnO/C/Co composite separately. It was observed that the nano ZnO/C /Co composite decolorizes the dye faster than nano ZnO/C composite and nano ZnO. It was also observed that about 66 % of the dye degraded within 10 min in the presence of nano ZnO/C/Co composite, where as in the presence of pristine nano ZnO and nano ZnO/C composites the degradation of the dye was found to be 27 % and 53% respectively as shown in figure 5.
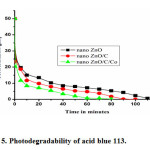 |
Figure5: Photodegradability of acid blue 113. Click here to View figure |
The higher degradation of nano ZnO/C/Co composite may be attributed to the higher surface area of the catalyst and an enhanced adsorption due to carbon which is a prerequisite for photodegradation [27]. The preliminary results suggest that nano ZnO/C/Co composite can be used as effective photocatalyst for the degradation of dye acid blue 113.
Conclusions
The nano ZnO/C/Co composite was found to be a more effective catalyst towards the degradation of acid blue 113 when compared to pristine nano ZnO particle and nano ZnO/C composites. The optimum carbon loading in the catalyst ZnO/C/Co was maintained at 10%. The dye removal was found to be maximum at a pH 6.9. The optimum catalyst loading was found to be 0.5 g L-1. The required time for 98% decolorization of the one liter of solution containing 50 μM of dye at pH 6.9 was 80 min.
References
- Environmental Protection Agency, US Environmental protection agency report DC. 2007: EPA100/B-07/001, EPA Washington
- Rajkumar, D.; Jong, G. K. J. Haz. Materials. 2006, 136, 203
- Kansal, S. K; Singh, M; Sud, D; J. Haz. Materials. 2007, 141(3), 581
- Gouvea, C. A. K; Wypych, F; Moraes, S. G; Duran, N; Nagata, N; Zamora, P. P. Chemosphere. 2000, 40(4), 433
- Neppolian, B.; Choi, H. C.; Sakthivel, S.; Banumathi, A.; Murugesan, V. J. Haz. Materials. 2002, 89, 303
- Cristian, L.; Juanita, F.; Jaime, B.; Mansilla, H. D. Catalysis.today. 2002, 76(2-4), 235
- Rajat, A.; Jitendra, V.; Punjabi, P. B.; Ameta, S. C. Indian J. Chem. Tech. 2006, 13, 114
- Sivalingam, G.; Nagaveni, K.; Hegde, M. S.; Giridhar, M. Applied Catalysis B: Envir. 2003, 45, 23
- Junping, L.; Yao, X.; Yong, L.; Dong, W.; Yuhan, S. China Particuolog. 2004, 2, 266
- Changchun, C.; Jiangfeng, L.; Ping, L.; Benhai, Y. Advances in Chem. Engin. and Scien. 2011, 1, 9
- Suwanboon, S.; Amornpitoksuk, P.; Haidoux, A.; Tedenac, J. C. J. Alloys Compd. 2008, 462, 335
- Zu, P.; Tang, Z. K.; Wong, G. K. L.; Kawasaki, M.; Ohtomo, A, Koinuma, K.; Sagawa. Solid State Commun. 1997, 103, 459
- Curri, M. L. Mater. Sci. Eng, 2003, 23, 285
- Muruganandham, M.; Wu, J. J. Appl. Catal. B: Environ. 2008, 80, 32
- Daneshvar, N.; Salari, D.; Khataee, A. R. J. Photochem. and Photobiol. A: Chem. 2004, 162, 317
- Noraidura, A.; Rusmidah, A.; Wan, A.; Wan, A. B.; Mohamed, N. G.; Mohemed, Y. O. Jurnal. Teknologi. 2007, 47, 1
- Comparelli, R.; Fanizza, E.; Curri, M. L.; Cozzi, P. D.; Mascolo, G.; Agostiano, A. Appl. Catal. B. 2005, 60,1
- Zhang, L. W.; Cheng, H. Y.; Zong, R. L.; Zhu, Y. F. J. Phys. Chem. C. 2009, 113, 2368
- Fu, H. B.; Xu, T. G.; Zhu, Y. F. Environ. Sci. Technol, 2008, 42, 8064
- Wang, Z. C. ACS Nano. 2008, 2, 1987
- Xu, L.; Au, Y.; Pelligra, C.; Chen, C.; Jin, L.; Huang, H.; Sithambaram, S.; Aindow, M.; Joeshon, R.; Suib, S. L. Chem. Mater. 2009, 21, 2875
- Saleh, R.; Djaja, N. F. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscoy. 2014, S 1386-1425(14)00500-9
- Nocera, D. G. Inorg. Chem. 2009, 48, 10001
- Duan, X.; Huang, Y.; Agrawal, R.; Lieber, C. M. Nature, 2003, 1, 241
- Jaya lakshmi, M.; Palaniappa, M.; Balasubramanian, K. Int. J. Electrochem. Science. 2008, 3, 96
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. Nano Res, 2011, 4(11), 1144
- Muthirulan, P.; Meenakshisundararam, M.; Kannan, N. J. Adv. Res, 2013, 4, 479

This work is licensed under a Creative Commons Attribution 4.0 International License.









