Synthesis of Macrocyclic Ionophore for the Development of Highly Selective Chloride Sensor
Sameena Mehtab*, Tanveer Irshad Siddiqi
Department of Chemistry, Lovely Professional University, Punjab-144806, India
DOI : http://dx.doi.org/10.13005/ojc/310121
Article Received on :
Article Accepted on :
Article Published : 03 Mar 2015
The macrocyclic ligands 3,8,12,17-tetramethyl-2,18,9,11-bipyridyl-1,4,7,10,13,16-hexaazacyclooctadecanetetrahydro bromide has been synthesized and explored as suitable ionophores for chloride selective membrane sensors. It displays Nernstian behavior (59.2 mV decade-1) across the range of 4.1 × 10-8 to 1.0 × 10-2 M. The detection limit of the electrode is ~ 15 nM and the response time and life times are 14 s and eight weeks respectively over a wide pH range (3.5 - 9.5). Interference from other anions is very low and it can be used as indicator electrode in the potentiometric titration of chloride ions and to determine chloride in agricultural soil water samples.
KEYWORDS:Chloride Membrane Sensor; Ion-Selective Electrode; Macrocyclic Ionophore
Download this article as:| Copy the following to cite this article: Mehtab S, Siddiqi T. I. Synthesis of Macrocyclic Ionophore for the Development of Highly Selective Chloride Sensor. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Mehtab S, Siddiqi T. I. Synthesis of Macrocyclic Ionophore for the Development of Highly Selective Chloride Sensor. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7548 |
Introduction
The determination of the concentrations of ionic species in aqueous samples is important in many areas of applied analytical chemistry, e.g., in process control, and in the analysis of clinical, horticultural, or environmental samples. The ion-selective electrodes are of widespread interest as they provide a rapid, accurate, reproducible, selective, and low-cost analytical method of specific guests1. ISEs are also of obvious interest because they can help translate the chemistry of new substrate binding systems into tools that can be used to recognize selectively various targeted species in the presence of potentially interfering analytes. Anions show a wide range of shapes and geometries and a lot of them are pH-sensitive, factors that have to be taken into account when designing a specific anion host. Anion receptors thus use binding forces that typically involve hydrogen bonding, electrostatic interactions and coordination to suitable metal centre. The effective anion-selective electrode should incorporate a selective ionophore for a guest anion in its membrane matrix2.
Among inorganic anions, Cl– anion is particularly important as it plays an important role in a variety of biological3 and physiological processes4, clinical diagnosis5, environmental monitoring6 and industrial applications7-9. Furthermore, its metabolic imbalance in blood and other body fluids results in various diseases and pathological disorders such as stroke, edema, cystic fibrosis, and atherosclerosis. With a continuous emergence of numerous ionophores, great progress has been made in the research in the direct detection of chloride concentration and potentiometric titration. As an electro active material in an electrode, the ionophore plays a crucial role in determining the performance characteristics of ion selective sensors. Some anion exchangers10,11 have been used as Cl– anion-selective ionophores but often lacked selectivity toward Cl– anion over other lipophilic anions such as NO3– and ClO4–. Lewis acid macrocycles12 and lanthanide tris(β-diketonates) have also been utilized to prepare Cl– anion-selective electrodes that suffered from significant interference from I–, Br, and SCN– anions13,14. Several other strategies are followed to design an ionophore with variety of functionalities which can compliment with the shape and size of an anion thereby showing selectivity towards a particular anion, like, 2,5-dihydroxy-p-benzoquinone15, Polypyrrole-graphite- epoxy composite16, Amino methylated polystyrene salicylaldehyde schiff base Co(II) complex17, Calix[4]arene derivative with ureido moiety18, Tridodecylmethyl ammonium chloride19,20, hydrogen bond forming ionophores21,22, and epoxy resins23. Though many species have been examined as potential chloride ionophores, only few have yielded requisite chloride selectivity to be used in the preparation of membrane electrode.
For better performance the ionophore ought to preferentially bind with the target ion and should possess a high degree of its own photochemical and redox stability. Therefore, it is not surprising that the robust macrocycles and their metal derivatives, due to their rich coordination chemistry and structural diversity, have got much attention as ionophores for the selective recognition and sensing of cations and anions24-29. They are incorporated, as ion carrier agents, in suitable polymeric membranes to get the ion selective membrane electrodes. These electrodes respond selectively to the concentration changes of a particular cation or anion and thereby transduce the analyte ion activity into a potential read-out.
Objective of the present paper is to synthesize a new highly selective ionophore and to optimize membrane compositions to achieve a chloride ion sensor of improved response characteristics. The 3,8,12,17-Tetramethyl-2,18,9,11-Bipyridyl-1,4,7,10,13,16-Hexaaza Cyclooctadecane Tetrahydro bromide (Scheme. 1) represent a class of macrocycle that should act as anion binding agents30,31. In this anion-receptor interactions of macrocyclic ionophore are expected to reflect contributions from both electrostatic and hydrogen bonding interactions in varying degrees depending on the conditions (e.g., degree of protonation, nature of solvent, etc.) Because of this potential duality, it was proposed that studies of appropriately chosen analytes, capable of multiple types of supra molecular interactions, would provide invaluable insights into the fundamental molecular recognition properties of this class of receptor.
Experimental
Reagents
Reagent grade hexadecyl trimethylammonium bromide (HTAB), dibutylphthalate (DBP), benzyl acetate (BA), dioctylphthalate (DOP), tributyl phosphate (TBP), dioctyl phthalate (DOP), o-nitrophenyloctyl ether (o-NPOE), tetrahydrofuran (THF), barium chloride dihydrate (BaCl2.2H2O) sodium borohydride (NaBH4), ethylenediamine, hydrobromic acid, and high molecular weight poly(vinylchloride) were purchased from E. Merck (Germany) and used as received. 2,6-Diacetylpyridine and methylacrylate acrylonitrile were procured from S. D. Fine-chem. Ltd. Sodium salts of all anions used were of analytical grade and used without any further purification. The solutions of anion salts were prepared in milliipore water and standardized whenever necessary.
Apparatus
Carbon, hydrogen, and nitrogen were analyzed with a Vario EL III elemental analyzer after carefully dried samples under vacuum for several hours. The UV–Vis spectra were recorded on Perkin–Elmer Lambda 35 UV/Vis spectrophotometer. IR spectra were obtained on a Thermo Nikolet Nexus FT-IR spectrometer in KBr. Room temperature magnetic susceptibility measurements were done on a Princeton applied research vibrating sample magnetometer Model. All potential measurements were performed at ambient temperature using a Century scientific-digital pH/ millivolt-meter.
Synthesis and Characterization of 3,8,12,17-Tetramethyl-2,18,9,11-Bipyridyl-1,4,7,10,13,16-Hexaaza Cyclooctadecane Tetrahydrobromide as Macrocyclic Ionophore
To synthesize macrocyclic ionophore (MI) using well established 2+2 template condensation method, followed by NaBH4 reduction of the imine as shown in the scheme 1. Ba(II) was chosen as the templating ion due to its success in promoting template condensation of macrocycles of this ring size. This adaption of the synthetic scheme is yield optimized (65%) and convenient in that condensation and the reduction step was performed in situ. A solution of ethylene diamine (0.5 mL, 7.5 mmol) in a minimum amount of methanol (10 mL) was added drop wise over 15 minutes to the stirred solution of 2,6-diacetyl pyridine (1.224 g, 7.5 mmol) and BaCl2.2H2O (0.915 g, 3.75 mmol). The mixture was refluxed for 12 h. Yellow solution was allowed to cool to room temperature. Solid NaBH4 (0.751 g, 20 mmol) was added slowly and the flask was placed in an ice bath and was stirred for 15 minutes. A second addition of NaBH4 (0.375 g, 10 mmol) was done and the resulting yellow-white mixture was stirred at room temperature for one hour. Evaporation of solvent under reduced pressure yielded a yellow-white product, which was extracted with dichloromethane (DCM). Evaporation of combined extracts to dryness yielded orange oil. This oil was dissolved in 20 mL of methanol, and 48 % hydrobromic acid (about 6 mL) was added until pH paper tested strongly acidic.
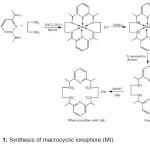 |
Scheme1: Synthesis of macrocyclic ionophore (MI). Click here to View scheme |
The product was recrystallised by dissolving in a minimum amount of hot water to which was added a small amount of activated carbon. After the mixture was filtered, absolute ethanol was added to incipient cloudiness while the heating and stirring were maintained. The solution was cooled to room temperature and placed in a refrigerator for two days. The white crystalline solid (MI) was washed with ice cold ethanol and stored in a vacuum desiccators. Yield 46 %; M.P. > 3000C; color white crystalline solid, reaction time 12 h; Anal. Calcd for C22H38Br4N6: C, 37.42; H, 5.42; Br, 45.26; N, 11.90. Found: C, 37.25; H, 5.49; N, 11.82. IR (KBr, cm-1) υ max: 3732, 2351, 2324, 1824, 1740, 1615, 1442, 1155, 1021 cm-1; 1H NMR (CDCl3, TMS, δ ppm, 500 MHz): 7.882 (t, 2H), 7.428 (d, 4H), 1.580 (s, 12H), 3.404 (qt, 8H), 4.698 (m, 4 H); 13C NMR (CDCl3, δ ppm, 500 MHz): 14.53, 40.29, 57.37, 120.85, 139.07, 153.26; UV-Visble: 202, 260 nm.
Preparation of Membranes
In the present work ion sensitive membranes of the PVC matrix were fabricated according to the procedure described by Craggs et al.32. An important requirement for making PVC membranes of an ion selective sensor is that the ionophore, PVC and plasticizers and anionic additives should be soluble in some fast evaporating solvent. The ionophores, PVC, different plasticizers and anionic additives used in the present investigations were found to be sufficiently soluble in THF. Therefore, the solvent THF was used for preparing membranes. Thus, membrane cocktails were formulated by dissolving appropriate amounts of ionophores, anionic additives and plasticizers. The components were added in terms of weight percentages. After thorough dissolution, the homogeneous mixture was concentrated by evaporating THF and it was then poured into polyacrylates rings placed on a smooth glass plate. The solution should be poured gently so that bubbles are not formed. A pad of filter papers and a heavy weight are then placed on top of the ring and the assembly was left for one day to allow the tetrahydrofuran to evaporate slowly at room temperature. After 24 hours of evaporation, the transparent membranes of 0.4 mm thickness were removed carefully from the glass plate and glued to one end of pyrex glass tube. It is known that the sensitivity, linearity and selectivity of ion selective sensor depend significantly on the membrane composition. Thus, optimization was performed after a good deal of experimentation to provide membranes, which generate reproducible and stable potentials. The membranes having only PVC as membrane ingredient (dummy membranes) have also been prepared to observe whether any background potentials being produced due to binding material or not.
Preparation of Sandwich Membranes
Ion-selective electrode membranes were cast from above mentioned procedure. The blank membranes (without ionophore) were also prepared having same composition. The sandwich membrane was made by pressing two individual membranes (ordinarily one without ionophore and one with the same components and an additional ionophore) together immediately after blotting them individually dry with tissue paper. The obtained sandwich membrane was visibly checked for air bubbles before mounting in electrode body with the ionophore-containing segment facing the sample solution. The combined segmented membrane was then rapidly mounted on to the electrode body and immediately measured33,34.
Potential Measurement
For the determination of chloride ion, membranes were equilibrated for 48 h in 0.01 M of NaCl solutions. The performance of the electrode was investigated by measuring its potential in NaCl solutions in the concentration range 1.0 ´ 10-9 – 1.0 ´ 10-2 M. Small concentrations of NaCl was obtained by gradual dilution of 0.01 M NaCl solution with millipore water. The potential measurements were carried out at 25 ± 1oC using following cell assemblies:
Ag/AgCl | KCl(satd) | test solution || PVC membrane || 0.01 M NaCl| Hg/Hg2Cl2 | KCl(satd)
Results and Discussion
Response of Different Anions
In preliminary experiments, various PVC-membrane ion-selective electrodes with the synthesized macrocyclic ionophore (MI) were prepared and tested for different anions. The potential response of the electrodes based on MI for different anions are shown in Fig 1. The results exhibited significantly high selectivity to chloride ion over other anions. Hence, the macrocyclic ionophore (MI) was selected as a carrier for preparation of chloride sensor.
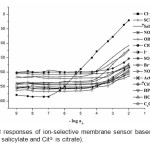 |
Figure1: Potential responses of ion-selective membrane sensor based MI for various anions *(Sal– is salicylate and Cit3- is citrate). Click here to View figure |
The Effect of Membrane Composition on Potential Response of the Chloride Sensor
Potential of the membranes of MI investigated as a function of chloride ion activity in the range 1.0 × 10-9 to 1.0 × 10-2 M and the results obtained are compiled in Table 1. The electrodes with no carrier (containing PVC, plasticizer and HTAB) displayed insignificant sensitivity towards chloride. The influence of plasticizer on the response characteristics of the chloride electrodes was investigated by using five plasticizers of different polarities including DBP, o-NPOE, DOP, BA and TBP. The sensor no. 3 having membrane without plasticizer exhibits a narrow working concentration range of 10-4 to 10-2 M with a sub Nernstian slope. Improvement in sensors performance was observed by the addition of plasticizer. Among the several membranes tested for each of the carriers, the membranes incorporating DOP showed better potentiometric responses, i.e., higher sensitivity and wider linearity of the calibration plots (sensor no. 11, Table 1).
The addition of lipophilic cationic additive in anion selective membranes is necessary to introduce perm selectivity. The influence and concentration of the membrane additives was also investigated by incorporating HTAB into the membranes. The potentiometric sensitivity of the membranes based on both carriers was greatly improved in the presence of HTAB as a lipophilic cationic additive, compared to the membranes with no additive at all. Previous studies have shown that there is an optimal concentration of lipophilic ionic additives in the membranes and that gives the best electrode performance35. The effect of HTAB concentration in the membrane was investigated at several additive/ionophore mole ratios. The sensors with HTAB/MI mole ratios of ~ 0.55 for both of the carriers exhibited maximum sensitivity over a wide range of chloride concentration.
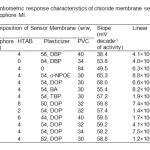 |
Table1: Potentiometric response characteristics of chloride membrane sensors based on ionophore MI. Click here to View table |
The Dynamic Response Time and Life Time of the Proposed Electrode
Dynamic response time is an important factor for chloride sensitive sensors. In this study, the practical response time has been recorded (for sensor no. 11) by changing solutions with different Cl– concentrations. The measurement sequence was from the lower (1.0 × 10−8 M) to the higher (1.0 ×10−2 M) concentration. The resulting data shows that the time needed to reach a potential with in ± 1.0 mV of the final equilibrium value after successive immersion of a series of Cl– solution, each having a tenfold difference in concentration is only 14 s.
To evaluate the reversibility of the electrode, a similar procedure in the opposite direction was adopted. The measurements have been performed in the sequence of high-to-low from (1.0 ´ 10-2 – 1.0 ´ 10-6 M) sample concentrations. The results showed that, the potentiometric response of the electrodes was reversible; although the time needed to reach equilibrium values (34 s) was longer than that of low-to high sample concentrations. The life time of proposed sensor is around eight weeks as potentiometric characteristics of the sensor remain almost constant with in this period of time.
Calibration Characteristics
Among the different membrane compositions, membranes with MI/HTAB/DOP/PVC in (w/w, %) 10/4/54/32 showed highest sensitivity and widest linear range and were selected as the optimum composition for further studies (Fig. 2). These sensors exhibit the maximum working concentration range of 4.1 × 10-8 to 1.0 × 10-2 M, detection limit 1.5 × 10−8 M with a slope of 59.2 ± 0.2 mVdecade-1 of activity (Sensor no. 11, Table 1). Repeated monitoring of potentials and calibration, using the same electrode over several days gave good slope reproducibility; the standard deviation (S.D.) of slope was 0.2 mV decade−1 activity (N=3).
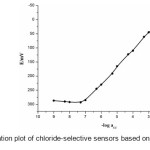 |
Figure2: Calibration plot of chloride-selective sensors based on MI ionophore. Click here to View figure |
The Influence of pH on Electrode’s Parameters
The pH dependence of the electrode potential was investigated in over the pH range of 1-12 for 10-2 M, 10-3 M and 10-4 M of Cl– ion solutions. The potentiometric response of the chloride sensor was found to be sensitive to pH changes. pH was adjusted by the addition of 0.01 M nitric acid and sodium hydroxide. The results given in Fig. 3 show that the potential response remains constant over the pH range ~3.5 – 9.5 and the same can be taken as the working pH range of the electrode. The significant change in potential response observed at a deviation at pH values < 3.5, can be due to simultaneous response of the electrode to the oppositely charged H3O+ and Cl– ions. The contribution of H3O+ to potential response counteracts that of Cl– ions. The observed potential drift at high pH values could be due to response of sensor to OH– ions.
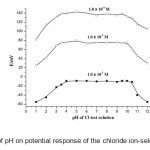 |
Figure3: Effect of pH on potential response of the chloride ion-selective sensors. Click here to View figure |
Anions Effect on Sensor Selectivity
Selectivity is an important characteristic of a sensor that delineates the extent to which the device may be used in the estimation of analyte ion in the presence of other ions and extent of utility of any sensor in real sample measurement. In this work, the selectivity coefficients of the sensors toward different anionic species (An-) were evaluated by using both the matched potential method (MPM)36,37 and the fixed interference method (FIM)38.
In the MPM, the selectivity coefficient () was determined by measuring the change in potential upon increasing the primary ion (Cl–) activity from an initial value of aCl to a΄Cl and aB represents the activity of interfering ion added to the reference solution of primary ion of activity aCl which also brings about same potential change. It is given by expression:

In the present studies aCl and a΄Cl were kept at 1.0 × 10−4 and 1.0 × 10−3 M Cl– and aB was experimentally determined. FIM is the most widely used procedure as per IUPAC recommendation for determining selectivity coefficients39. In the FIM, the selectivity coefficient was evaluated from potential measurement on solutions containing a fixed concentration of interfering ion (1.0 × 10-2 M) and varying amount of Cl– ions. The values of selectivity coefficient so determined are compiled in Table 2.
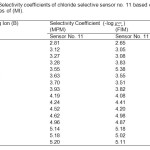 |
Table2: Selectivity coefficients of chloride selective sensor no. 11 based on the membranes of (MI). Click here to View table |
A value of selectivity coefficient equal or greater than to 1.0 indicates equal response to both primary ion and interfering ions. A value smaller than 1.0 shows that the sensor is selective to the primary ion over the interfering ion. It is seen from the Table 2, that the selectivity coefficients determined by both the methods are sufficiently smaller than 1.0 indicating that the present sensors are significantly selective to chloride ion over all the interfering ions. Most of the interfering ions showed low values of selectivity coefficients (~ 10−3 – 10−4), indicating no or minimum interference in the performance of the electrode assembly.
Determination of Formation Constant
Formation constant of the ion–ionophore complex within the membrane phase is a very important parameter that dictates the practical selectivity of the sensor. In this method, two membrane segments are fused together, with only one containing the ionophore, to give a concentration-polarized sandwich membrane. A membrane potential measurement of this transient condition reveals the ion activity ratio at both interfaces, which translates into the apparent binding constants of the ion-ionophore complex40. In this method complex formation constants obtained by neglecting ion pairing because ion-pairing effects are relatively nonspecific i.e, all ion pair formation constants have approximately the same value. As reported, the membrane potential EM is determined by subtracting the cell potential for a membrane without ionophore from that for the sandwich membrane. The formation constant is then calculated from the following equation 2:
Where LT is the total concentration of ionophore in the membrane segment, RT is the concentration of lipophilic ionic site additives, n is the ion-ionophore complex stoichiometry, and R, T and F are the gas constant, the absolute temperature, and the Faraday constant. The ion carries a charge of zI. The determined formation constants (log βILn) for the examined different complexes were recorded in Table 3. The elapsed time between sandwich fusion and exposure to electrolyte was typically <1 min. The potential was recorded as the mean of the last minute of a 5 min measurement period in the appropriate salt solution. The potential of such sandwich membranes remains free of diffusion-induced potential drifts for about 20 min. Standard deviations were obtained based on the measurements of sets of at least three replicate membrane disks that were made from the same parent membrane. A careful analysis of the data in Table 3 reveals that chloride ion has significant anion-binding characteristics.
![Table 3. Formation constant values of various ions for Cl− selective membrane based on [MI].](http://www.orientjchem.org/wp-content/uploads/2015/03/Vol31_No1_Synt_SAMEE_T3-150x150.jpg) |
Table3: Formation constant values of various ions for Cl− selective membrane based on [MI]. Click here to View table |
Analytical Applications
The proposed chloride membrane sensor (no. 11) was found to work well under laboratory conditions. It was applied successfully as an indicator electrode in the potentiometric titration of 20 mL of 1.0 × 10-4 M NaCl with 1.0 × 10-3 M AgNO3 and the resulting titration curve are shown in Fig 4. As can be seen, the amount of chloride can be determined with sensor.
The analytical utility and sensitivity of the proposed membrane sensor was verified by analysis of Cl– ion from soil water samples. Four basic factors determine the amount of Cl– available to crops growing
in well drained soils, the Cl– concentration in the soil solution; atmospheric deposition of Cl–; the Cl– concentration in the irrigation water; and the content of Cl– in fertilizers and manure. As a result of their genetic characteristics, crops have different requirements and tolerance to Cl–. The amount of Cl– added to a field via the irrigation water (fresh or treated municipal effluents) depends on farm activities. Water of low to medium salinity contains 100 – 300 g Cl– m-3, whereas saline water contains 300 – 1200 g Cl– m-3(41-44).
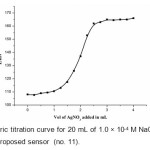 |
Figure4: Potentiometric titration curve for 20 mL of 1.0 × 10-4 M NaCl with 1.0 × 10-3 M AgNO3 using the proposed sensor (no. 11). Click here to View figure |
The direct potentiometric measurement was carried out using standard addition technique, where recovery percentage in the range 94.4 – 97.3. The result obtained for the determination of chloride ion at several concentrations is in close agreement with the known chloride content as given in Table 4. Recovery experiments were performed by repeated measurements (n = 3) of soil water samples containing various amounts of chloride ions.
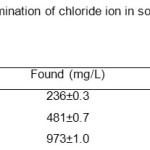 |
Table 4. Potentiometric determination of chloride ion in soil water samples (mean ± S.D., n = 3). Click here to View table |
 |
Table5: Comparison of the proposed Cl–-selective electrodes (sensor no. 11) with the reported electrode. Click here to View table |
Conclusions
We synthesize a new macrocycle as anion-recognizing ionophore 3,8,12,17-Tetramethyl-2,18,9,11-Bipyridyl-1,4,7,10,13,16-Hexaaza Cyclooctadecane Tetrahydrobromide for the fabrication of potentiometric membrane and fully characterize the potentiometric performances of selective chloride ion membrane sensor. The proposed macrocylcic ionophore is found to be very useful in ISE membranes for chloride determination. Compared with earlier sensors, the new ISE for Cl– shows advantages as to stability, lower detection limit, high pH range and good selectivity. Fast response and a reasonable long-term stability to chloride ions characterize the proposed electrode. On the basis of their selectivities, this sensor is expected to be adequate for the environmental water monitoring. Present sensor no. 11 was compared with some reported chloride selective sensors (Table 5). It is seen that the selectivity, working concentration range and pH range of the proposed sensor toward chloride is better as compared to reported sensors.
Acknowledgement
Dr. Sameena Mehtab and Dr. Tanveer Irshad Siddiqi are grateful to Council of Scientific and Industrial Research, New Delhi, India for providing financial assistance for this work.
References
- Morf, W. E.; The Principles and Ion-selective Electrodes and Membrane Transport, Elsevier, Amsterdam 1981.
- Bakker, E.; Buhlmann, P.; Pretsch, E. Chem. Rev. 1997, 97, 3083-3132
- Oka, S.; Sibaraki, Y.; Tahara, S. Anal. Chem. 1991, 53, 588-593
- Seiler, H. G.; Sigel, H.; Sigel, A.; Handbook on Toxicity of Inorganic Compounds; Dekker: New York 1988.
- Huber, C.; Werner, T.; Krause, C.; Klimant, I.; Wolfbeis, O. S. Anal. Chim. Acta 1998, 364, 143-151
- Martin, A.; Narayanaswamy, R. Sens. Actuators B 1997, 39, 330-333
- Badr, I. H. A.; Diaz, M.; Hawthorne, M. F.; Bachas, L. G. Anal. Chem. 1999, 71, 1371-1377
- Geddes, C. D. Sens. Actuators B 2001, 72, 188-195
- Ashcroft, F. M. Ion Channels and Diseases, Academic Press: San Diego, C A, 2000
- Oka, S.; Sibazaki, Y.; Tahara, S. Anal. Chem. 1981, 53, 588-593
- Ozawa, S.; Miyag, H.; Shibata, Y.; Oki, N.; Kunitake, T.; Keller, W. E. Anal. Chem. 1996, 68, 4149-4152
- Badr, I. H. A.; Diaz, M.; Hawthorne, M. F.; Bachas, L. G. Anal. Chem. 1999, 71, 1371-1377
- Dzhabarov, V. I.; Knyazev, A. A.; Yu, G. G. Russian, J. Appl. Chem. 2006, 79, 1816-1819
- Mahajan, R. K.; Kaur, I. K. R.; Onimaru, A.; Shinoda, S.; Tsukube, H. Anal. Chem. 2004, 76, 7354-7359
- Sundaram, R.; Hariprasad, K. S. Ind J. Chem. Soc. 2007, 14, 451-456
- lvarez-Romero, G. A.; Ramrez-Silva, M. T.; Galan-Vidal, C.A.; Paez-Hernandez, M. E.; Romero-Romoc, M. A. Electroanalysis 2010, 22; 1650-1654
- Kumar, K. G.; John, K. S.; Indra, C. J. Ind J. Chem. Soc. 2006, 13, 13-17
- Babu, J. N.; Bhalla, V.; Kumar, M.; Mahajan, R. K.; Puri, R. K. Tetrahedron Letters 2008, 49, 2772-2775
- Legin, A.; Makarychev-Mikhailov, S.; Kirsanov, D.; Mortensen, J.; Vlasov, Y. Anal. Chim. Acta 2004, 514, 107-113
- Lyczewska, M.; Wojciechowski, M.; Bulska, E.; Elizabeth, A. H.; Hall, K.; Maksymiuk, A.; Michalska, Electroanalysis 2007, 19, 393-397
- Xiao, K. P.; Bühlmann, P.; Nishizawa, S.; Amemiya, S.; Umezawa, Y. Anal. Chem. 1997, 69, 1038-1044
- Gupta, V. K.; Goyal, R. N.; Sharma, R. A. Electrochim. Acta 2009, 54, 4126-4132
- Shin, J. H.; Lee, H. L.; Cho, S. H.; Ha, J.; Nam, H.; Cha, G. S. Anal. Chem. 2004, 76, 4217-4222
- Singh, A. K.; Mehtab, S.; Saxena, P. Talanta, 2006, 69; 1143-1148
- Kumar, A.; Mehtab, S.; Singh, U. P.; Aggarwal, V.; Singh, J. Electroanalysis, 2008, 20, 1186-1193
- Simonneaux, G.; Maux, P. L. Coord. Chem. Rev. 2002, 228, 43-60
- Biesaga, M.; Pyrzynska, K.; Trojanowicz, M. Talanta 2000, 51, 209-224
- Chou, J. H.; Kosal, M. E.; Nalwa, H. S.; Rakow, N. A.; Suslick, K. S.; Kadish, K.; Smith, K.; Guilard R. (Eds.), The Porphyrin Handbook, Vol. VI; Academic Press, New York, 2000
- Purrello, R.; Gurrieri, S.; Lauceri, R. Coord. Chem. Rev. 1999, 190, 683-706
- Fibbioli, M.; Berger, M.; Schmidtchen, F. P.; Pretsch, E. Anal. Chem. 2000, 72, 156-160
- Beer, P. D.; Schmitt, P. Curr. Bio. Chem. Bio. 1997, 1, 475-482
- Craggs, A.; Keil, L.; Moody, G.J.; Thomas, J. D. R. Talanta 1975, 22, 907-910
- Mi, Y.; Bakker, E. Anal. Chem. 1999, 71, 5279-5287
- Perez, M. A.; Marin, L. P.; Quintana, J. C.; Pedram, M. Y. Sens. Actuators B, 2003, 89, 262-268
- Schaller, U.; Bakker, E.; Spichiger, U. E.; Pretsch E. Anal. Chem. 1994, 66, 391-398
- Umezawa, Y.; Umezawa, K.; Sato, H. Pure Appl. Chem. 1995, 67, 507-518
- Gadzekpo, V. P. Y.; Christian, G. D. Anal. Chim. Acta 1984, 164, 279-282
- Bakker, E.; Pretsch, E.; Bühlmann, P.; Anal. Chem. 2000, 72, 1127-1133
- Viteri, F. J. S.; Diamond, D. Analyst, 1994, 119, 749-758
- Shultz, M. M.; Stefanova, O. K.; Mokrov, S. B.; Mikhelson, K. N. Anal. Chem. 2002, 74, 510-517
- Goos, R. J. Crops Soils 1987, 39, 12-13
- LaCroix, R. L.; Keeney, D. R.; Walsh, L. M. Commun. Soil Sci. Plant Anal., 1970, 1, 1-6
- Narayanan, P. N. V. Handbook of Ammonium Chloride Fertilizer, McMillan, India, 1990
- Krieg, D. R.; Sung, D. Commun. Soil Sci. Plant Anal. 1977, 8, 109-114

This work is licensed under a Creative Commons Attribution 4.0 International License.










