Synthesis, Characterization and Biological Evaluation of Some Novel Thiophene Anchored Fluorinated Heterocycles
B. K. Karale1*, H. N. Akolkar1, A. S. Burungale2, S. D. Mhaske1 and R. S. Endait1
1P. G. Department of Chemistry, Radhabai Kale Mahila Mahavidyalaya, Ahmednagar, 414001, India.
2Post-Graduate and Research Center in Chemistry, S. M. Joshi College, Hadapsar, Pune-28
DOI : http://dx.doi.org/10.13005/ojc/310155
Article Received on :
Article Accepted on :
Article Published : 08 Feb 2015
A new series of thiophene anchored 1,3,4-thiadiazole, 1,2,4-triazole, 2-heteryl chromone, 1,5-benzothiazepine, pyrazoline, 2-styryl chromone derivatives containing fluorine are synthesized, characterized by spectral methods and screened them for various biological activities.
KEYWORDS:1;3;4-Thiadiazole; 1;2;4-Triazole; 2-Heteryl chromone; 1;5-Benzothiazepine; Pyrazoline; 2-Styryl chromone
Download this article as:| Copy the following to cite this article: Karale B. K, Akolkar H. N, Burungale A. S, Mhaske S. D, Endait R. S. Synthesis, Characterization and Biological Evaluation of Some Novel Thiophene Anchored Fluorinated Heterocycles. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Karale B. K, Akolkar H. N, Burungale A. S, Mhaske S. D, Endait R. S. Synthesis, Characterization and Biological Evaluation of Some Novel Thiophene Anchored Fluorinated Heterocycles. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7060 |
Introduction
Thiophene is sulfur containing five membered heterocyclic compound widely used as building block in agrochemicals1. Thiophene containing compounds exhibit antimicrobial2, antiparasitic3, anticancer4 and anticonvulsant 5 activities.
2-Styryl chromones are associated with various pharmacological activities such as antiallergic6, cytotoxic7, antioxidant8, anti-inflammatory9 and antibacterial10. 2-Styryl chromones are also acts as β-amyloid imaging agents11. Pyrazole derivatives are known for various potent biological activities such as antibacterial12, antioxidant12, anticancer13, ACE inhibitor14.
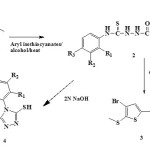 |
Scheme 1 Click here to View scheme |
Thiadiazole derivatives possess antitumor15, antiinflammatory16, SIRT1 inhibitor17, antihypertensive18, antimicrobial19 activities. 1,2,4-Triazole anchored compounds are associated with antimicrobial19, antioxidant20, anti-inflammatory20, CYP enzyme inhibitor21, anxiolytic22 activities.
Compounds containing chromone scaffold are acts as breast cancer resistance protein ABCG2 inhibitor23, monoamine oxidase inhibitor24, adenosine A2A receptor antagonists25. Some 1,5-benzothiazepines are antifungal26, anticonvulsant27, anti breast cancer28, antithrombotic29, antidepressant30 agents. Compounds having pyrazoline moity are known to possess anti-inflammatory31, antimalarial32, antitubercular33, antidepressant34 activities.
The activities associated with these various heterocycles prompted us to synthesize some novel thiophene anchored fluorinated heterocycles.
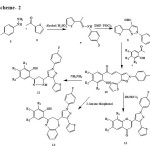 |
Scheme 2 Click here to View scheme |
Biological Activities
Antimicrobial activity
Synthesized compounds were screened for their antifungal and antibacterial activities. The in vitro antimicrobial activities of the synthesized compounds were assessed against fungi and bacteria. The fungi used were C. albicans, A. Fumigatus and A. Niger. The bacterias used were S. aureus, E. coli, S. Epidermidis and P. Vulgaris.
 |
Scheme 3 Click here to View scheme |
Fluconazole and Amikacin were used as standards for comparison for antifungal and antibacterial activities respectively. The activities were determined by measuring the diameter of the inhibition zone in mm.
None of the compounds is a suitable candidate for antifungal and antibacterial indication as shown in Table 2.
Experimental
Melting points were recorded in open capillaries in liquid paraffin bath and are uncorrected. IR spectra were recorded on Perkin-Elmer FTIR spectrophotometer in KBr disc.1H NMR spectra were recorded on Bruker 400 MHz NMR spectrometer in DMSO as a solvent and TMS as an internal standard. Peak values are shown in δ (ppm). Mass spectra were recorded on Finnigan mass spectrometer.
2-{[4-Bromo-5-(methylsulfanyl)thiophen-2-yl]carbonyl}-N-phenylhydrazinecarbothioamide 2.
Equimolar amounts (0.01 mole) of compound 1 and aryl isothiocyanate were dissolved in 15 mL of ethanol. The reaction mixture was heated under reflux for 55 minutes. The progress of reaction was monitored by TLC. After completion of reaction the contents were cooled and the solid obtained was filtered and crystallized from ethanol to get compound 2.
2a
IR (KBr, cm-1): 3327, 3228, 3113, 1643, 1257; 1H NMR (DMSO): δ 2.22 (s, 3H), 3.3 (s, 3H), 7.00-7.20 (m, 4H), 7.8 (s, 1H), 9.5 (s, 2H), 10.6 (s, 1H); MS: m/z 430 (M+); Elemental Anal. Calcd.: C, 38.89; H, 3.26; N, 9.72; found: C, 38.91; H, 3.29; N, 9.74 %.
2b
IR (KBr, cm-1): 3324, 3220, 3117, 1640, 1262; 1H NMR (DMSO): δ 2.21 (s, 3H), 7.04-7.21 (m, 4H), 7.87 (s, 1H), 9.54 (s, 2H), 10.64 (s, 1H); MS: m/z 418 (M+); Elemental Anal. Calcd.: C, 37.15; H, 2.64; N, 10.00; found: C, 37.18; H, 2.67; N, 10.04 %.
2c
IR (KBr, cm-1): 3330, 3224, 3109, 1647, 1255; 1H NMR (DMSO): δ 2.21 (s, 3H), 7.03-7.20 (m, 4H), 7.86 (s, 1H), 9.53 (s, 2H), 10.62 (s, 1H); MS: m/z 434 (M+); Elemental Anal. Calcd.: C, 35.75; H, 2.54; N, 9.62; found: C, 35.78; H, 2.57; N, 9.65 %.
2d
IR (KBr, cm-1): 3328, 3225, 3111, 1641, 1257; 1H NMR (DMSO): δ 2.22 (s, 3H), 7.00-7.25 (m, 5H), 7.83 (s, 1H), 9.52 (s, 2H), 10.65 (s, 1H); MS: m/z 400 (M+); Elemental Anal. Calcd.: C, 38.81; H, 3.01; N, 10.44; found: C, 38.84; H, 3.05; N, 10.48 %.
2e
IR (KBr, cm-1): 3330, 3224, 3119, 1651, 1264; 1H NMR (DMSO): δ 2.20 (s, 3H), 3.34 (s, 3H), 7.05-7.24 (m, 4H), 7.81 (s, 1H), 9.53 (s, 2H), 11.01 (s, 1H); MS: m/z 430 (M+); Elemental Anal. Calcd.: C, 38.89; H, 3.26; N, 9.72; found: C, 38.92; H, 3.29; N, 9.74 %.
5-(4-Bromo-5-(methylthio)thiophen-2-yl)-N-phenyl-1,3,4-thiadiazol-2-amine 3
Thiosemicarbazide 2 (0.001 mole) was dissolved in 3 mL of conc. H2SO4 in 50 mL beaker. The reaction mixture was stirred at room temperature for 3 hr. After completion of reaction 10 g of crushed ice was added in it. The solid obtained was separated by filtration and crystallized from 1:1 mixture of DMF and water to afford thiadiazole 3.
3a
IR (KBr, cm-1): 3161, 1633, 1591; 1H NMR (DMSO): δ 2.3 (s, 3H), 3.5 (s, 3H), 6.9-7.4 (m, 4H), 7.8 (s, 1H), 10.9 (s, 1H); MS: m/z 412 (M+); Anal. Calcd.: C, 40.58; H, 2.92; N, 10.14; found: C, 40.60; H, 2.94; N, 10.17 %.
3b
IR (KBr, cm-1): 3166, 1630, 1587; 1H NMR (DMSO): δ 2.31 (s, 3H), 6.95-7.82 (m, 5H), 10.94 (s, 1H); MS: m/z 400 (M+); Anal. Calcd.: C, 38.81; H, 2.25; N, 10.44; found: C, 38.84; H, 2.28; N, 10.47 %.
3c
IR (KBr, cm-1): 3156, 1638, 1598; 1H NMR (DMSO): δ 2.30 (s, 3H), 6.91-7.83 (m, 5H), 10.91 (s, 1H); MS: m/z 416 (M+); Anal. Calcd.: C, 37.28; H, 2.17; N, 10.03; found: C, 37.31; H, 2.20; N, 10.06 %.
3d
IR (KBr, cm-1): 3167, 1632, 1588; 1H NMR (DMSO): δ 2.31 (s, 3H), 6.92-7.89 (m, 6H), 10.93 (s, 1H); MS: m/z 382 (M+); Anal. Calcd.: C, 40.63; H, 2.62; N, 10.93; found: C, 40.66; H, 2.65; N, 10.96 %.
3e
IR (KBr, cm-1: 3164, 1629, 1592; 1H NMR (DMSO): δ 2.3 (s, 3H), 3.52 (s, 3H), 6.91-7.86 (m, 4H), 10.9 (s, 1H); MS: m/z 412 (M+); Anal. Calcd.: C, 40.58; H, 2.92; N, 10.14; found: C, 40.61; H, 2.95; N, 10.17 %.
5-(4-Bromo-5-(methylthio)thiophen-2-yl)-4-phenyl-4H-1,2,4-triazole-3-thiol 4.
A mixture of thiosemicarbazide 2 and 10 mL of 1N NaOH was heated under mild reflux for 1.5 hr. The progress of reaction was monitored by TLC. After completion of reaction the contents were cooled and poured into crushed ice. Then it was acidified with glacial acetic acid. The product was separated by filtration and crystallized from the mixture (1:1) of DMF and water to get corresponding triazole 4.
4a
IR (KBr, cm-1): 3070, 2997, 1583, 1517; 1H NMR (DMSO): δ 2.0 (s, 3H), 3.5 (s, 3H), 6.4-7.5 (m, 5H), 14.11 (s, 1H); MS: m/z 412 (M+); Anal. Calcd.: C, 40.58; H, 2.92; N, 10.14; found: C, 40.61; H, 2.95; N, 10.17 %.
4b
IR (KBr, cm-1): 3077, 2990, 1578, 1514; 1H NMR (DMSO): δ 2.01 (s, 3H), 6.49-7.56 (m, 5H), 14.12 (s, 1H); MS: m/z 400 (M+); Anal. Calcd.: C, 38.81; H, 2.25; N, 10.44; found: C, 38.84; H, 2.28; N, 10.47 %.
4c
IR (KBr, cm-1): 3081, 2983, 1588, 1521; 1H NMR (DMSO): δ 2.01 (s, 3H), 6.48-7.58 (m, 5H), 14.11 (s, 1H); MS: m/z 416 (M+); Anal. Calcd.: C, 37.28; H, 2.17; N, 10.03; found: C, 37.32; H, 2.20; N, 10.07; %.
4d: IR (KBr, cm-1): 3085, 2990, 1580, 1517; 1H NMR (DMSO): δ 2.01 (s, 3H), 6.4-7.57 (m, 6H), 14.1 (s, 1H); MS: m/z 382 (M+); Anal. Calcd.: C, 40.63; H, 2.62; N, 10.93; found: C, 40.67; H, 2.65; N, 10.96 %.
4e
IR (KBr, cm-1): 3074, 2991, 1589, 1510; 1H NMR (DMSO): δ 2.02 (s, 3H), 3.55 (s, 3H), 6.46-7.59 (m, 5H), 14.10 (s, 1H); MS: m/z 412 (M+); Anal. Calcd.: C, 40.58; H, 2.92; N, 10.14; found: C, 40.62; H, 2.95; N, 10.18;%.
(E)-3-(1-(4-Fluorophenyl)-3-(thiophen-2-yl)-1H-pyrazol-4-yl)-1-(2-hydroxyphenyl)prop-2-en-1-one 10.
Equimolar amount of compound 8 (0.02 mole) and substituted o-hydroxy acetophenone (0.02 mole) were dissolved in 25 mL of alcohol in conical flask. To this reaction mixture 40% KOH (10mL) was added. The reaction mixture was stirred at room temperature for 48 hrs. The contents were then poured into crushed ice and neutralized with acetic acid. The yellow solid thus obtained was filtered and crystallized from alcohol to afford compound 10.
10a
IR (KBr, cm-1): 3134 (O-H), 1637 (C=O), 1568 (C=N), 1556 (-C=C-), 1508 (-C=C-, aromatic), 1155 (Ar-F); 1H NMR (DMSO- d6): d 7.26- 8.22 (m, 11H, Ar-H and =CH), 9.44 (s, 1H), 13.2 (s, 1H, -O-H); MS: m/z 458 (M+);; Anal. Calcd.: C, 57.53; H, 2.85; N, 6.10; found: C, 57.56; H, 2.88; N, 6.13 %.
10b
IR (KBr, cm-1): 3132 (O-H), 1633 (C=O), 1566 (C=N), 1549 (-C=C-), 1511 (-C=C-, aromatic), 1158 (Ar-F); 1H NMR (DMSO- d6): d 7.25- 8.34 (m, 12H, Ar-H and =CH), 9.47 (s, 1H), 13.21 (s, 1H, -O-H); MS: m/z 424 (M+); Anal. Calcd.: C, 62.19; H, 3.32; N, 6.59; found: C, 62.21; H, 3.35; N, 6.62%.
10c
IR (KBr, cm-1): 3129 (O-H), 1631 (C=O), 1569 (C=N), 1556 (-C=C-), 1510 (-C=C-, aromatic), 1157 (Ar-F); 1H NMR (DMSO- d6): d 7.27- 8.24 (m, 12H, Ar-H and =CH), 9.45 (s, 1H), 13.22 (s, 1H, -O-H); MS: m/z 468 (M+); Anal. Calcd.: C, 56.30; H, 3.01; N, 5.97; found: C, 56.34; H, 3.05; N, 6.01 %.
10d
IR (KBr, cm-1): 3130 (O-H), 1637 (C=O), 1571 (C=N), 1551 (-C=C-), 1501 (-C=C-, aromatic), 1149 (Ar-F); 1H NMR (DMSO- d6): d 2.2 (s, 3H), 7.25- 8.22 (m, 11H, Ar-H and =CH), 9.46 (s, 1H), 13.20 (s, 1H, -O-H); MS: m/z 438 (M+); Anal. Calcd.: C, 62.94; H, 3.67; N, 6.38; found: C, 62.98; H, 3.70; N, 6.41 %.
10e
IR (KBr, cm-1): 3134 (O-H), 1641 (C=O), 1561 (C=N), 1555 (-C=C-), 1509 (-C=C-, aromatic), 1155 (Ar-F); 1H NMR (DMSO- d6): d 2.24 (s, 3H), 2.29 (s, 3H), 7.27- 8.23 (m, 11H, Ar-H and =CH), 9.44 (s, 1H), 13.2 (s, 1H, -O-H); MS: m/z 418 (M+); Anal. Calcd.: C, 68.88; H, 4.58; N, 6.69; found: C, 68.92; H, 4.62; N, 6.73 %.
10f
IR (KBr, cm-1): 3140 (O-H), 1635 (C=O), 1567 (C=N), 1547 (-C=C-), 1499 (-C=C-, aromatic), 1146 (Ar-F); 1H NMR (DMSO- d6): d 2.24 (s, 3H), 7.21- 8.26 (m, 12H, Ar-H and =CH), 9.45 (s, 1H), 13.20 (s, 1H, -O-H); MS: m/z 404 (M+); Anal. Calcd.: C, 68.30; H, 4.24; N, 6.93; found: C, 68.34; H, 4.28; N, 6.97%.
2-(1-(4-Fluorophenyl)-3-(thiophen-2-yl)-1H-pyrazol-4-yl)-4H-chromen-4-one 11.
Compound 10 (0.001 mole) was dissolved in 15 ml DMSO. To this reaction mixture catalytic amount of iodine (0.01 gm) was added. The reaction mixture was heated to 100 to 1100C for 1.5 hrs and left overnight. Then 100 ml ice cold water was added in it. The solid thus obtained was filtered and washed with dil. sodium thiosulphate followed by water. The product was crystallized from alcohol to afford compounds 11.
11a
IR (KBr, cm-1): 1655 (C=O), 1567 (C=N), 1513 (C=C), 1158 (Ar-F), 1H NMR (DMSO- d6): d 6.77 (s, 1H, Ar-H), 7.14-8.16 (m, 9H, Ar-H), 9.22 (s, 1H); MS: m/z 456 (M+); Anal. Calcd.: C, 57.78; H, 2.42; N, 6.13; found: C, 57.82; H, 2.46; N, 6.17 %.
11b
IR (KBr, cm-1): 1649 (C=O), 1561 (C=N), 1508 (C=C), 1156 (Ar-F), 1H NMR (DMSO- d6): d 6.71 (s, 1H, Ar-H), 7.13-8.18 (m, 10H, Ar-H), 9.24 (s, 1H); MS: m/z 422 (M+); Anal. Calcd.: C, 62.49; H, 2.86; N, 6.62; found: C, 62.53; H, 2.89; N, 6.65%.
11c
IR (KBr, cm-1): 1651 (C=O), 1562 (C=N), 1516 (C=C), 1149 (Ar-F), 1H NMR (DMSO- d6): d 6.73 (s, 1H, Ar-H), 7.15-8.13 (m, 10H, Ar-H), 9.23 (s, 1H); MS: m/z 466 (M+); Anal. Calcd.: C, 56.54; H, 2.59; N, 5.99; found: C, 56.57; H, 2.62; N, 6.02 %.
11d
IR (KBr, cm-1): 1659 (C=O), 1571 (C=N), 1510 (C=C), 1148 (Ar-F), 1H NMR (DMSO- d6): d 6.77 (s, 1H, Ar-H), 7.15-8.19 (m, 9H, Ar-H), 9.23 (s, 1H); MS: m/z 436 (M+); Anal. Calcd.: C, 63.23; H, 3.23; N, 6.41; found: C, 63.27; H, 3.27; N, 6.45%.
11e
IR (KBr, cm-1): 1645 (C=O), 1569 (C=N), 1513 (C=C), 1155 (Ar-F), 1H NMR (DMSO- d6): d 2.23 (s, 3H), 2.29 (s, 3H), 6.79 (s, 1H, Ar-H), 7.13-8.15 (m, 9H, Ar-H), 9.21 (s, 1H); MS: m/z 416 (M+); Anal. Calcd.: C, 69.21; H, 4.11; N, 6.73; found: C, 69.25; H, 4.14; N, 6.76 %.
11f
IR (KBr, cm-1): 1650 (C=O), 1562 (C=N), 1507 (C=C), 1146 (Ar-F), 1H NMR (DMSO- d6): d 2.23 (s, 3H), 6.78 (s, 1H, Ar-H), 7.14-8.17 (m, 10H, Ar-H), 9.23 (s, 1H); MS: m/z 402 (M+); Anal. Calcd.: C, 68.64; H, 3.76; N, 6.96; found: C, 68.68; H, 3.79; N, 6.99 %.
2-(5-(1-(4-Fluorophenyl)-3-(thiophen-2-yl)-1H-pyrazol-4-yl)-4,5-dihydro-1H-pyrazol-3-yl)phenol 12.
Compound 10 (0.003 mol) was taken in 100 mL RBF with 15 mL dioxane. To this reaction mixture 1 mL hydrazine hydrate was added and the contents were heated under refluxed for 4 hours. Then to the reaction mixture 2 mL gl. acetic acid was and heating was continued for further 3 hours. After complete heating contents were cooled to room temperature and poured over crushed ice. The solid thus obtained was separated by filtration and crystallized with alcohol to get compounds 12. Products obtained were identified with help of spectral data. Their characterization data is given in the Table-1.
Table 1 – Characterization data of synthesized compounds
| Compd | R1 | R2 | R3 | m.p.(0C) | Yield (%) | Compd | R1 | R2 | R3 | m.p.(0C) | Yield (%) |
| 2a | H | OCH3 | H | 186 | 65 | 12a | Cl | H | Cl | 190 | 67 |
| 2b | H | H | F | 180 | 74 | 12b | H | H | Cl | 171 | 63 |
| 2c | H | H | Cl | 184 | 71 | 12c | H | H | Br | 194 | 70 |
| 2d | H | H | H | 179 | 73 | 12d | H | CH3 | Cl | 178 | 69 |
| 2e | OCH3 | H | H | 155 | 61 | 12e | CH3 | H | CH3 | 156 | 62 |
| 3a | H | OCH3 | H | 180 | 60 | 12f | H | CH3 | H | 130 | 59 |
| 3b | H | H | F | 199 | 65 | 13a | Cl | H | Cl | 230 | 73 |
| 3c | H | H | Cl | 172 | 73 | 13b | H | H | Cl | 188 | 78 |
| 3d | H | H | H | 177 | 60 | 13c | H | H | Br | 200 | 74 |
| 3e | OCH3 | H | H | 190 | 62 | 13d | H | CH3 | Cl | 208 | 71 |
| 4a | H | OCH3 | H | 235 | 68 | 13e | CH3 | H | CH3 | 232 | 69 |
| 4b | H | H | F | 209 | 72 | 13f | H | CH3 | H | 164 | 64 |
| 4c | H | H | Cl | 222 | 56 | 15a | Cl | H | Cl | 178 | 62 |
| 4d | H | H | H | 240 | 60 | 15b | H | H | Cl | 154 | 67 |
| 4e | OCH3 | H | H | 200 | 66 | 15c | H | H | Br | 168 | 66 |
| 10a | Cl | H | Cl | 218 | 72 | 15d | H | CH3 | Cl | 160 | 61 |
| 10b | H | H | Cl | 186 | 69 | 15e | H | H | CH3 | 142 | 58 |
| 10c | H | H | Br | 178 | 66 | 16a | Cl | H | Cl | 250 | 69 |
| 10d | H | CH3 | Cl | 158 | 69 | 16b | H | H | Cl | 270 | 63 |
| 10e | CH3 | H | CH3 | 160 | 68 | 16c | H | H | Br | 274 | 68 |
| 10f | H | CH3 | H | 160 | 63 | 16d | H | CH3 | Cl | 234 | 60 |
| 11a | Cl | H | Cl | 258 | 62 | 16e | H | H | CH3 | 165 | 52 |
| 11b | H | H | Cl | 264 | 68 | 17a | Cl | H | Cl | 275 | 62 |
| 11c | H | H | Br | 254 | 67 | 17b | H | H | Cl | 295 | 65 |
| 11d | H | CH3 | Cl | 258 | 69 | 17c | H | H | Br | 284 | 60 |
| 11e | CH3 | H | CH3 | 238 | 65 | 17d | H | CH3 | Cl | 298 | 63 |
| 11f | H | CH3 | H | 196 | 61 | 17e | H | H | CH3 | 272 | 61 |
12a
IR (KBr, cm-1): 3405 (-O-H), 3320 (-N-H), 3071 (Ar-H), 1587 (C=N); 1H NMR (DMSO): δ 3.20 (dd, 1H), 3.74 (dd, 1H), 5.1 (t, 1H), 7.17-8.13 (m, 10H, aromatic and N-H protons), 8.58 (s, 1H, pyrazole proton), 12.00 (s, 1H, -O-H proton); MS: m/z 472 (M+); Anal. Calcd.: C, 55.82; H, 3.19; N, 11.84; found: C, 55.86; H, 3.23; N, 11.88 %.
12b
IR (KBr, cm-1): 3409 (-O-H), 3325 (-N-H), 3074 (Ar-H), 1580 (C=N); 1H NMR (DMSO): δ 3.21 (dd, 1H), 3.74 (dd, 1H), 5.11 (t, 1H), 7.16-8.14 (m, 11H, aromatic and N-H protons), 8.57 (s, 1H, pyrazole proton), 12.01 (s, 1H, -O-H proton); MS: m/z 438 (M+); Anal. Calcd.: C, 60.20; H, 3.67; N, 12.77; found: C, 60.24; H, 3.71; N, 12.80 %.
12c
IR (KBr, cm-1): 3402 (-O-H), 3322 (-N-H), 3070 (Ar-H), 1581 (C=N); 1H NMR (DMSO): δ 3.22 (dd, 1H), 3.74 (dd, 1H), 5.12 (t, 1H), 7.11-8.12 (m, 11H, aromatic and N-H protons), 8.59 (s, 1H, pyrazole proton), 12.04 (s, 1H, -O-H proton); MS: m/z 482 (M+); Anal. Calcd.: C, 54.67; H, 3.34; N, 11.59; found: C, 54.71; H, 3.38; N, 11.63 %.
12d
IR (KBr, cm-1): 3410 (-O-H), 3320 (-N-H), 3072 (Ar-H), 1577 (C=N); 1H NMR (DMSO): δ 2.23 (s, 3H), 3.21 (dd, 1H), 3.72 (dd, 1H), 5.11 (t, 1H), 7.16-8.16 (m, 10H, aromatic and N-H protons), 8.57 (s, 1H, pyrazole proton), 12.02 (s, 1H, -O-H proton); MS: m/z 452 (M+); Anal. Calcd.: C, 60.99; H, 4.01; N, 12.37; found: C, 61.03; H, 4.05; N, 12.41 %.
12e
IR (KBr, cm-1): 3407 (-O-H), 3324 (-N-H), 3065 (Ar-H), 1579 (C=N); 1H NMR (DMSO): δ 2.23 (s, 3H), 2.25 (s, 3H), 3.23 (dd, 1H), 3.73 (dd, 1H), 5.12 (t, 1H), 7.16-8.14 (m, 10H, aromatic and N-H protons), 8.56 (s, 1H, pyrazole proton), 12.01 (s, 1H, -O-H proton); MS: m/z 432 (M+); Anal. Calcd.: C, 66.65; H, 4.89; N, 12.95; found: C, 66.69; H, 4.93; N, 12.99 %.
12f
IR (KBr, cm-1): 3401 (-O-H), 3325 (-N-H), 3066 (Ar-H), 1591 (C=N); 1H NMR (DMSO): δ 2.24 (s, 3H), 3.21 (dd, 1H), 3.72 (dd, 1H), 5.13 (t, 1H), 7.15-8.13 (m, 11H, aromatic and N-H protons), 8.55 (s, 1H, pyrazole proton), 12.00 (s, 1H, -O-H proton); MS: m/z 418 (M+); Anal. Calcd.: C, 66.01; H, 4.58; N, 13.39; found: C, 66.05; H, 4.62; N, 13.43 %.
2-((E)-2-(1-(4-Fluorophenyl)-3-(thiophen-2-yl)-1H-pyrazol-4-yl)-2,3-dihydrobenzo[b][1,4]thiazepin-4-yl)phenol 13.
Compound 10 (0.001 mol) and o-amino thiophenol (0.001 mol) was suspended in 10 ml ethanol. Reaction mass was heated to reflux at 900C for 4-5h. To the reaction mixture 2 ml glacial acetic acid was added and heating continued for further 3h. Contents were cooled and poured into crushed ice. The product obtained was separated by filtration and crystallized from ethanol. Products obtained were identified with help of spectral data. Their characterization data is given in the Table-1.
13a: IR (KBr): 3413 (-O-H), 3066 (Ar-H), 1590 & 1566 (C=N), 612 (C-S) cm-1; 1H NMR (DMSO): δ 3.12 (t, 1H), 3.76 (dd, 1H), 5.57 (dd, 1H), 7.18-8.05 (m, 13H, Ar-H), 8.51 (s, 1H, pyrazole proton), 15.9 (s, 1H, -O-H proton); MS: m/z 565 (M+); Anal. Calcd.: C, 59.36; H, 3.20; N, 7.42; found: C, 59.40; H, 3.23; N, 7.46;%.
13b: IR (KBr): 3411 (-O-H), 3070 (Ar-H), 1594 & 1570 (C=N), 611 (C-S) cm-1; 1H NMR (DMSO): δ 3.11 (t, 1H), 3.75 (dd, 1H), 5.57 (dd, 1H), 7.17-8.04 (m, 14H, Ar-H), 8.50 (s, 1H, pyrazole proton), 15.95 (s, 1H, -O-H proton); MS: m/z 531 (M+); Anal. Calcd.: C, 63.21; H, 3.60; N, 7.90; found: C, 63.25; H, 3.64; N, 7.94 %.
13c: IR (KBr): 3409 (-O-H), 3062 (Ar-H), 1597 & 1562 (C=N), 616 (C-S) cm-1; 1H NMR (DMSO): δ 3.12 (t, 1H), 3.77 (dd, 1H), 5.54 (dd, 1H), 7.16-8.07 (m, 14H, Ar-H), 8.52 (s, 1H, pyrazole proton), 15.93 (s, 1H, -O-H proton); MS: m/z 575 (M+); Anal. Calcd.: C, 58.33; H, 3.32; N, 7.29; found: C, 58.37; H, 3.36; N, 7.33 %.
13d: IR (KBr): 3407 (-O-H), 3068 (Ar-H), 1590 & 1568 (C=N), 611 (C-S) cm-1; 1H NMR (DMSO): δ 2.24 (s, 3H), 3.10 (t, 1H), 3.73 (dd, 1H), 5.57 (dd, 1H), 7.17-8.07 (m, 13H, Ar-H), 8.50 (s, 1H, pyrazole proton), 15.92 (s, 1H, -O-H proton); MS: m/z 545 (M+); Anal. Calcd.: C, 63.78; H, 3.88; N, 7.69; found: C, 63.82; H, 3.92; N, 7.73 %.
13e: IR (KBr): 3410 (-O-H), 3066 (Ar-H), 1594 & 1565 (C=N), 610 (C-S) cm-1; 1H NMR (DMSO): δ 2.24 (s, 3H), 2.26 (s, 3H), 3.13 (t, 1H), 3.74 (dd, 1H), 5.56 (dd, 1H), 7.16-8.01 (m, 13H, Ar-H), 8.53 (s, 1H, pyrazole proton), 15.91 (s, 1H, -O-H proton); MS: m/z 525 (M+); Anal. Calcd.: C, 68.55; H, 4.60; N, 7.99; found: C, 68.59; H, 4.64; N, 8.03 %.
13f: IR (KBr): 3404 (-O-H), 3062 (Ar-H), 1584 & 1559 (C=N), 610 (C-S) cm-1; 1H NMR (DMSO): δ 2.21 (s, 3H), 3.12 (t, 1H), 3.74 (dd, 1H), 5.57 (dd, 1H), 7.14-8.03 (m, 14H, Ar-H), 8.54 (s, 1H, pyrazole proton), 15.90 (s, 1H, -O-H proton); MS: m/z 511 (M+); Anal. Calcd.: C, 68.08; H, 4.33; N, 8.21; found: C, 68.11; H, 4.37; N, 8.24 %.
(E)-2-Acetylphenyl 3-(1-(4-fluorophenyl)-3-(thiophen-2-yl)-1H-pyrazol-4-yl)acrylate 15.
Equimolar amount (0.05 moles) of the compounds 14 and substituted 2-hydroxy acetophenone were taken in dry beaker. To this mixture was dissolved in 15 ml dry pyridine. The reaction mixture was then cooled to 00C. To this reaction mixture POCl3 (0.06 moles) was added drop wise maintaining temperature below 100C. Then reaction mixture was kept overnight at room temperature. It was then poured over crushed ice with vigorous stirring. Product was separated by filtration, washed with ice-cold water and then with 2% ice-cold solution of NaOH followed by ice-cold water again. Purification by crystallization after drying with alcohol afforded 15.
15a
IR (KBr, cm-1): 3120 (O-H), 1736 (C=O), 1549 (C=N), 1545 (-C=C-), 1499 (-C=C-, aromatic), 1147 (Ar-F); 1H NMR (DMSO- d6): d 2.21 (s, 3H), 7.05- 8.30 (m, 11H, Ar-H and =CH), 8.44 (s, 1H, pyrazole proton); MS: m/z 500 (M+); Anal. Calcd.: C, 57.50; H, 3.02; N, 5.59; found: C, 57.54; H, 3.06; N, 5.63 %.
15b
IR (KBr, cm-1): 3129 (O-H), 1734 (C=O), 1554 (C=N), 1544 (-C=C-), 1494 (-C=C-, aromatic), 1143 (Ar-F); 1H NMR (DMSO- d6): d 2.20 (s, 3H), 7.08- 8.35 (m, 12H, Ar-H and =CH), 8.48 (s, 1H, pyrazole proton); MS: m/z 466 (M+); Anal. Calcd.: C, 61.74; H, 3.45; N, 6.00; found: C, 61.78; H, 3.48; N, 6.03 %.
15c
IR (KBr, cm-1): 3124 (O-H), 1739 (C=O), 1558 (C=N), 1558 (-C=C-), 1506 (-C=C-, aromatic), 1142 (Ar-F); 1H NMR (DMSO- d6): d 2.21 (s, 3H), 7.03- 8.31 (m, 12H, Ar-H and =CH), 8.47 (s, 1H, pyrazole proton); MS: m/z 510 (M+); Anal. Calcd.: C, 56.37; H, 3.15; N, 5.48; found: C, 56.40; H, 3.18; N, 5.52 %.
15d
IR (KBr, cm-1): 3130 (O-H), 1732 (C=O), 1551 (C=N), 1549 (-C=C-), 1495 (-C=C-, aromatic), 1141 (Ar-F); 1H NMR (DMSO- d6): d 2.20 (s, 3H), 7.03- 8.37 (m, 11H, Ar-H and =CH), 8.49 (s, 1H, pyrazole proton); MS: m/z 480 (M+); Anal. Calcd.: C, 62.43; H, 3.77; N, 5.82; found: C, 62.47; H, 3.80; N, 5.85 %.
15e
IR (KBr, cm-1): 3135 (O-H), 1739 (C=O), 1559 (C=N), 1535 (-C=C-), 1503 (-C=C-, aromatic), 1151 (Ar-F); 1H NMR (DMSO- d6): d 2.20 (s, 3H), 2.31 (s, 3H), 7.00- 8.27 (m, 12H, Ar-H and =CH), 8.42 (s, 1H, pyrazole proton); MS: m/z 446 (M+); Anal. Calcd.: C, 67.25; H, 4.29; N, 6.27; found: C, 67.28; H, 4.33; N, 6.30 %.
(E)-5-(1-(4-Fluorophenyl)-3-(thiophen-2-yl)-1H-pyrazol-4-yl)-1-(2-hydroxyphenyl)pent-4-ene-1,3-dione 16.
Compound 15 (0.03 moles) was dissolved in 15 ml of dry pyridine. To this mixture powdered KOH (1 gm) was added and the reaction mixture was stirred on the magnetic stirrer for 3 hours. Then it was poured over crushed ice and acidified with acetic acid. The product was then separated by filtration, washed with water, dried and crystallized with acetic acid to afford 16.
Table 2– Antimicrobial activity results for concentration 1000μg/ml.
| Compd |
Antifungal test models |
Antibacterial test models |
|||||
|
C. albicans
|
A. fumigatus
|
A. Niger |
S. aureus
|
E. coli
|
S. epidermidis |
P. Vulgaris |
|
| 2a | 12 | 8 | 10 | 12 | 11 | 12 | – |
| 2b | 10 | – | 12 | 10 | 8 | 10 | 8 |
| 2c | – | – | 10 | – | 10 | – | – |
| 2d | 10 | 8 | 8 | 12 | 6 | 12 | 8 |
| 2e | 10 | 6 | 8 | 11 | 10 | 10 | 8 |
| 3a | 8 | 6 | 8 | 10 | 11 | 8 | – |
| 3b | 10 | – | 6 | 12 | 10 | 8 | 12 |
| 3c | 6 | 8 | 6 | 8 | 12 | 6 | – |
| 3d | 11 | 6 | 8 | 8 | 10 | 8 | 6 |
| 3e | 8 | – | 6 | 11 | 8 | 6 | – |
| 4a | 10 | 8 | 8 | 12 | 10 | 8 | 6 |
| 4b | 10 | 6 | 4 | 10 | 10 | 6 | – |
| 4c | 12 | 6 | 6 | 11 | 10 | 8 | 4 |
| 4d | 10 | 8 | – | 10 | 6 | 6 | 8 |
| 4e | 8 | 4 | 6 | 12 | 10 | 8 | 4 |
| 10a | 8 | 4 | 10 | 8 | 8 | 6 | 8 |
| 10b | 4 | 6 | 8 | 6 | 4 | 4 | – |
| 10c | 8 | 6 | – | 4 | 6 | 8 | 6 |
| 10d | 6 | 4 | – | 10 | 8 | – | – |
| 10e | 10 | 6 | 4 | 6 | 8 | 9 | 8 |
| 10f | 4 | 6 | 4 | 8 | 6 | 6 | 4 |
| 11a | 8 | 6 | – | 6h | 8h | 6 | – |
| 11b | 6 | 8 | 6 | 12 | 10 | 11 | 12 |
| 11c | 11 | 6 | 8 | 10 | 6 | 8 | – |
| 11d | 10 | 6 | 4 | 6 | 8 | 11 | – |
| 11e | 8 | 4 | 6 | 8 | 6 | 6 | – |
| 11f | 4 | 8 | 6 | 6 | 7 | 4 | – |
| 12a | 11 | 10 | 8 | 6 | 4 | 6 | 8 |
| 12b | 10 | 5 | 6 | 4 | 6 | 6 | 4 |
| 12c | 10 | 6 | 6 | 12 | 10 | 6 | 6 |
| 12d | 8 | 6 | 6 | 10 | 8 | 10 | 8 |
| 12e | 10 | 6 | 6 | 12 | 8 | 6 | 12 |
| 12f | 8 | 4 | 6 | 10 | 6 | 8 | 4 |
| 13a | 10 | 6 | 8 | 10 | 8 | 6 | 6 |
| 13b | 6 | 8 | 6 | 8 | 6 | 8 | 4 |
| 13c | 8 | 10 | 10 | 6 | 8 | 10 | 6 |
| 13d | 8 | 4 | – | 8 | 10 | 8 | – |
| 13e | 6 | 8 | – | 8 | 10 | 6 | – |
| 13f | 6 | 7 | – | 8 | 6 | 10 | 6 |
| 15a | 8 | 8 | 6 | 10 | 8 | 6 | – |
| 15b | 6 | 6 | 4 | 9 | 6 | 8 | – |
| 15c | 8 | 6 | – | 10 | 6 | 4 | – |
| 15d | 8 | 8 | 8 | 4 | 6 | 10 | – |
| 15e | 8 | 4 | – | 4 | 7 | 8 | – |
| 16a | 12 | 8 | 4 | 6 | 8 | 10 | 4 |
| 16b | 12 | 10 | 8 | 6 | 4 | 6 | – |
| 16c | 8 | 6 | 6 | 12 | 10 | 13 | – |
| 16d | 10 | 8 | 6 | 12 | 10 | 10 | – |
| 16e | 10 | 8 | 6 | 10 | 8 | 6 | 6 |
| 17a | 10h | 8 | 6 | 6h | 8h | 8 | 4 |
| 17b | 12 | 8h | – | 10 | 12h | 10 | 8 |
| 17c | 12 | 6 | – | 8 | 12 | 6 | – |
| 17d | 6 | 4 | 6 | 7 | 9 | 6 | – |
| 17e | 12 | 6 | 8 | 11 | 14 | 16 | – |
| Fluconazole10µg/disc |
24 |
14 |
20 |
NA |
NA |
NA |
NA |
| Amikacin20µg/ml |
NA |
NA |
NA |
28 |
20 |
23 |
18 |
NT: Not tested, h: hazy.
16a
IR (KBr): 3094 (O-H), 1643 (C=O), 1623 (-C=C-), 1133 (Ar-F) cm-1; 1H NMR (DMSO- d6): δ 6.17-8.03 (m, 11H, Aromatic and olefinic protons), 11.54 (s, 1H, phenolic proton), 15.21 (1H, enolic proton); MS: m/z 500 (M+); Anal. Calcd.: C, 57.50; H, 3.02; N, 5.59; found: C, 57.54; H, 3.06; N, 5.63 %.
16b
IR (KBr): 3105 (O-H), 1649 (C=O), 1620 (-C=C-), 1140 (Ar-F) cm-1; 1H NMR (DMSO- d6): δ 6.11-8.10 (m, 12H, Aromatic and olefinic protons), 11.58 (s, 1H, phenolic proton), 15.20 (1H, enolic proton); MS: m/z 466 (M+); Anal. Calcd.: C, 61.74; H, 3.45; N, 6.00; found: C, 61.78; H, 3.49; N, 6.04 %.
16c
IR (KBr): 3087 (O-H), 1635 (C=O), 1620 (-C=C-), 1123 (Ar-F) cm-1; 1H NMR (DMSO- d6): δ 6.12-8.06 (m, 12H, Aromatic and olefinic protons), 11.55 (s, 1H, phenolic proton), 15.19 (1H, enolic proton); MS: m/z 510 (M+); Anal. Calcd.: C, 56.37; H, 3.15; N, 5.48; found: C, 56.40; H, 3.18; N, 5.51 %.
16d
IR (KBr): 3101 (O-H), 1652 (C=O), 1626 (-C=C-), 1142 (Ar-F) cm-1; 1H NMR (DMSO- d6): δ 2.31 (s, 3H), 6.20 -8.12 (m, 11H, Aromatic and olefinic protons), 11.49 (s, 1H, phenolic proton), 15.25 (1H, enolic proton); MS: m/z 480 (M+); Anal. Calcd.: C, 62.43; H, 3.77; N, 5.82; found: C, 62.47; H, 3.81; N, 5.86 %.
16e
IR (KBr): 3087 (O-H), 1633 (C=O), 1627 (-C=C-), 1147 (Ar-F) cm-1; 1H NMR (DMSO- d6): δ 2.20 (s, 3H), 6.23-8.01 (m, 12H, Aromatic and olefinic protons), 11.48 (s, 1H, phenolic proton), 15.21 (1H, enolic proton); MS: m/z 446 (M+); Anal. Calcd.: C, 67.25; H, 4.29; N, 6.27; found: C, 67.28; H, 4.32; N, 6.30 %.
2-((E)-2-(1-(4-Fluorophenyl)-3-(thiophen-2-yl)-1H-pyrazol-4-yl)vinyl)-4H-chromen-4-one 17.
Compound 16 (0.01 moles) was dissolved in 15 ml glacial acetic acid in RBF. To this reaction mixture 1 ml conc. HCl was added and contents were refluxed for 2 hours. Then it was cooled and poured over crushed ice. The product was then separated by filtration, washed with water, dried and crystallized with acetic acid to afford 17.
17a
IR (KBr, cm-1): 1651 (C=O), 1559 (C=N), 1505 (C=C), 1149 (Ar-F); 1H NMR (DMSO- d6): d 6.64 (s, 1H, Ar-H), 7.01-8.52 (m, 11H, Ar-H), 9.12 (s, 1H); MS: m/z 482 (M+); Anal. Calcd.: C, 59.64; H, 2.71; N, 5.80; found: C, 57.82; H, 2.46; N, 6.17 %.
17b
IR (KBr, cm-1): 1654 (C=O), 1548 (C=N), 1509 (C=C), 1156 (Ar-F); 1H NMR (DMSO- d6): d 6.65 (s, 1H, Ar-H), 7.07-8.40 (m, 12H, Ar-H), 9.16 (s, 1H); MS: m/z 448 (M+); Anal. Calcd.: C, 64.21; H, 3.14; N, 6.24; found: C, 64.24; H, 3.17; N, 6.27 %.
17c
IR (KBr, cm-1): 1659 (C=O), 1557 (C=N), 1501 (C=C), 1152 (Ar-F); 1H NMR (DMSO- d6): d 6.63 (s, 1H, Ar-H), 7.05-8.50 (m, 12H, Ar-H), 9.13 (s, 1H); MS: m/z 500 (M+); Anal. Calcd.: C, 58.43; H, 2.86; N, 5.68; found: C, 58.46; H, 2.89; N, 5.71 %.
17d
IR (KBr, cm-1): 1653 (C=O), 1549 (C=N), 1498 (C=C), 1141 (Ar-F); 1H NMR (DMSO- d6): d 2.31 (s, 3H), 6.65 (s, 1H, Ar-H), 7.01-8.42 (m, 11H, Ar-H), 9.15 (s, 1H); MS: m/z 462 (M+); Anal. Calcd.: C, 64.86; H, 3.48; N, 6.05; found: C, 64.90; H, 3.52; N, 6.09 %.
17e
IR (KBr, cm-1): 1658 (C=O), 1560 (C=N), 1514 (C=C), 1145 (Ar-F); 1H NMR (DMSO- d6): d 2.28 (s, 3H), 6.61 (s, 1H, Ar-H), 7.04-8.39 (m, 12H, Ar-H), 9.14 (s, 1H); MS: m/z 428 (M+); Anal. Calcd.: C, 70.08; H, 4.00; N, 6.54; found: C, 70.12; H, 4.03; N, 6.58 %.
References
- Ansary, A. K.; Omar, H. A. Bull. Faculty Pharm. 2001, 39, 17.
- Kavitha, P. N.; Vijayanthimala, P.; Saravanan, J.; Mohan, S. Res. J. Pharma., Bio. and Chem. Sci. 2010, 1(2), 124-130.
- Jose, L.; Gonzalez, C. E.; Stephens, T.; Wenzler, R. Eur. J. Med. Chem. 2007, 42, 552-557.
- Folkes, A. J.; Ahmadi, K.; Alderton, W. K.; Alix, S.; Baker, S. J. Journal of Medi. Chem. 2008, 51, 5522-5532.
- Kulandasamy, R.; Adhikari, A. V.; Stables, J. P. Eur. J. Med. Chem. 2009, 44(11), 4376-4384.
- Oganesyan, E. T.; Saraf, A. S.; Ivchenko, A. V. Pharma. Chem. Jour. 1993, 27(1), 52-54.
- Bhatnagar, S.; Sahi, S.; Kackar, P.; Kaushik, S.; Dave, M. K.; Shukla, A.; Goel, A. Bioorg. Med. Chem. Lett. 2010, 20(16), 4945–4950.
- Pawar, S. P.; Kondhare, D. D.; Zubaidha, P. K. Med. Chem. Res. 2013, 22 (2), 753- 757.
- Gomes, A.; Fernandes, E.; Silva, A. M. S.; Pinto, D. C. G. A.; Santos, C. M. M.; Cavaleiro, J. A.S.; Lima, J. L. F. C. Biochem. Pharmaco. 2009, 78(2), 171–177.
- Momin, M.; Ramjugernath, D.; Chenia, H.; Koorbanally, N. A. Journal of Chemistry 2013, Article ID 436758.
- Ono, M.; Maya, Y.; Haratake, M.; Nakayama, M. Bioorg. Med. Chem. 2007, 15, 444–450.
- Karale, B. K.; Pawar, P. Y.; Gadakh, A. V.; Akolkar, H. N.; Rindhe, S. S. Ind. Jour. Het. Chem. 2014, 23(3), 283-292.
- Peng-Cheng, Lv.; Hai-L, Z., Huan-Qiu, Li; Sun, J; Zhou, Y. Bioorg. Med. Chem. 2010, 18, 4606–4614.
- Bonesi, M; Loizzo, M. R.; Statti, G. A.; Michel, S.; Menichini, T. F. Bioorg. Med. Chem. Lett. 2010, 20, 1990–1993.
- Zhang, K.; Wang, P.; Xuan, L. N.; Fu, X. Y.; Jing, F.; Li, S.; Liu, Y. M.; Chen, B. Q. Bioorg. Med. Chem. Lett. 2014, 24 (22), 5154-5156.
- Jain, S. K.; Mishra, P. Eur. J. Exp. Bio. 2014, 4(2), 337-341.
- Wu, J.; Li, J.; Xu, M. H.; Liu, D. Bioorg. Med. Chem. Lett. 2014, 24(14), 3050-3056.
- Samel, A. B.; Pai, N. R. J. Chin. Chemi. Soc. 2010, 57, 1327-1330.
- Gadhave, A. G.; Gaikar, R. B.; Kuchekar, S. R.; Karale, B. K. Journal of Heterocyclic Chemistry 2014, 51(6), 1849–1855.
- Bhalgat, C. M.; Ali, M. I.; Ramesh, B.; Ramu, G. Arabian Journal of Chemistry 2014, 7, 986–993.
- Al-Soud, Y. A.; Heydel, M.; Hartmann, R. W. Tetrahedron Letters 2011, 52(48), 6372–6375.
- Tarzia, G.; Ocelli, E.; Toja, E.; Barone, D.; Corsico, N.; Gallico, L.; Luzzani, F. J. Med. Chem. 1988, 31(6), 1115-1123.
- Winter, E.; Lecerf-Schmidt, F.; Gozzi, G.; Peres, B.; Lightbody, M.; Gauthier, C.; Ozvegy-Laczka, C.; Szakacs, G.; Sarkadi, B.; Creczynski-Pasa, T. B.; Boumendjel, A.; Di Pietro, A. J. Med. Chem. 2013, 56(24), 9849-9860.
- Gaspar, A.; Silva, T.; Anez, M. Y.; Vina, D.; Orallo, F.; Ortuso, F.; Uriarte, E.; Alcaro, S.; Borges, F. J. Med. Chem. 2011, 54(14), 5165-5173.
- Andrews, S. P.; Mason, J. S.; Hurrell, E.; Congreve, M. Hide Affiliations Heptares Therapeutics Ltd., Biopark, Broadwater Road, Welwyn Garden City, UK E-mail: steve.andrews@heptares.com Med. Chem. Commun. 2014, 5, 571-575.
- Kang, W.; Du, X.; Wang, L.; Hu, L.; Dong, Y.; Bian, Y.; Li, Y. Chin. J. Chem. 2013, 31(10), 1305–1314.
- Garg, N.; Chandra, T.; Archana; Jain, A. B.; Kumar, A. Eur. J. Med. Chem. 2010, 45(4), 1529–1535.
- Ameta, K. L.; Rathore N. S.; Kumar, B. J. Serb. Chem. Soc. 2012, 77(6), 725– 731.
- Ayral, E.; Gloanec, P.; Berge, G.; de Nanteuil, G.; Mennecier, P.; Rupin, A.; Verbeuren, T. J.; Fulcrand, P.; Martinez, J.; Hernandez, J. F. Bioorg. Med. Chem. Lett. 2009, 19(5), 1386-1391.
- Tran, P. V.; Bymaster, F. P.; McNamara, R. K.; Potter, W. Z. J. Clin. Psychopharmacol. 2003, 23(1), 78-86.
- Sauzem, P. D.; Machado, P. M.; Rubin, A.; Sant’Anna, G. S.; Faber, H. B.; De Souza, A. H.; Mello, C. F.; Beck, P.; Burrow, R. A.; Bonacorso, H. G.; Zanatta, N.; Martins, M. A. P. Eur. J. Med. Chem. 2008, 43(6), 1237-1247.
- Cunico, W.; Cechinel, C. A.; Bonacorso, H. G.; Martins, M. A. P.; Zanatta, N.; De Souza, M. V. N.; Freitas, I. O.; Soares, R. P. P.; Krettli, A. U. Bioorg. Med. Chem. Lett. 2006, 16(3), 649-653.
- Ali, M. A.; Shaharyar, M. and Siddiqui, A. A., Eur. J. Med. Chem. 2007, 42(2), 268-275.
- Johnson, M.; Younglove, B.; Lee, L.; LeBlanc, R.; Holt, H.; Jr Hills, P.; Mackay, H.; Brown, T.; Mooberry, S. L.; Lee, M. Bioorg. Med. Chem. Lett. 2007, 17(21), 5897-5901.

This work is licensed under a Creative Commons Attribution 4.0 International License.









