Synthesis and Characterization of Some Fluorinated 1, 5 - Benzothiazepines and Pyrazolines
S. G. Jagadhani*, S. G. Kundlikar and B. K. Karale
P. G. and Research Department of Chemistry, Radhabai Kale Mahila Mahavidyalaya, Ahmednagar, 414 001, India.
DOI : http://dx.doi.org/10.13005/ojc/310177
Article Received on :
Article Accepted on :
Article Published : 31 Mar 2015
4-Bromo-2-fluorobenzaldehyde 1 when treated with substituted hydroxy acetophenones 2 yields chalcones 3. These chalcones were refluxed with 2-aminothiophenol gave “2-[2-(4-bromo-2-fluorophenyl)-2,3-dihydro-1,5-benzothiazepin-4-yl]phenol” 4 and when treated with hydrazine hydrate gave the compound “2-[5-(4-bromo-2-fluorophenyl)-4,5-dihydro-1H-pyrazol-3-yl]phenol” 5. The structures of compounds have been established on the basis of spectral data.
KEYWORDS:Fluorinated Chalcones; Benzothiazepines; Pyrazolines
Download this article as:| Copy the following to cite this article: Jagadhani S. G, Kundlikar S. G, Karale B. K. Synthesis and Characterization of Some Fluorinated 1,5 - Benzothiazepines and Pyrazolines. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Jagadhani S. G, Kundlikar S. G, Karale B. K. Synthesis and Characterization of Some Fluorinated 1,5 - Benzothiazepines and Pyrazolines. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=8177 |
Introduction
One of the most important factors in the drug design is that fluorine is much more lipophilic than hydrogen, so incorporating fluorine in the molecule makes it more fat soluble, so it percolates into the membrane more readily and hence fluorinated molecule has a higher bioavailability. Around fifth of all drugs on the market today contain at least one fluorine atom such as Paroxetine, Ezetimibe, Linezolid and Midazolam. Many fluorinated compounds are widely used as antimalarial, antiviral, antipsychotic and antidepressants. Some heterocyclic compounds also act as dyes, pesticides, luminophores and herbicides in nature1. Various biological activities associated with chalcones includes antimitotic2, antiinvasive3,4, antifungal5, antituberculosis6, antileishmanial7, anti- malarial8,9, antiinflammatory10-12, antitumor and antioxidant properties13. Their recognized synthetic utility in the preparation of pharmacologically interesting heterocycles as pyrazolines, which includes antiparasitary14, anti-tumor15, nitric oxide synthase inhibitors16 and anti-inflammatory17 activities.
Benzothiazepines retained the interest of researchers due to the unique structural properties and broad spectrum of biological activities of the compounds18. Three possible benzocondensed derivatives of 1,5-benzothiazepines viz. 1,4-, 4,1- and 1,5-benzothiazepines19 are kwown. Benzothiazepines have their role in the treatment of muscle relaxant20, cardiovascular disorders21, as Ca2+ channel blockers22 and inhibitors of HIV-integrase23. Pyrazolines reported to have anti-inflammatory24-26, anti-viral27, anti-cancer28-30, anti-diabetic31 and anti-oxidant32 properties. Several pyrazoline derivatives found to possess antimicrobial33 and anti-HIV34 activities. Some of the pyrazolines were effective in inhibiting the accumulation of prion protein35, the abnormal protease-resistant form.
Present Work
Substituted hydroxy acetophenones 2 on reaction with 4-bromo-2-fluorobenzaldehyde 1 stirred at room temperature for 24 hrs gives respective chalcones 3 which on reaction with 2-amino thiophenol & reflux for 8 hrs gave benzothiazepines 4 and with hydrazine hydrate for 6 hrs which gave pyrazolines 5.
Experimental
All the recorded melting points were determined in open capillary tubes and are uncorrected. I.R. spectra were recorded on Shimadzu FTIR Spectrophotometer in KBr disc. 1H NMR spectra were recorded on a Bruker Avance II 400 MHz spectrophotometer DMSO-d6 as a solvent and TMS as an internal standard (chemical shift in δ values). Mass spectra were obtained on a Finnigan mass spectrometer. Purity of the compounds was checked by TLC on silica gel G plates.
Synthesis of Chalcones
Compound 2 (0.005 mol) & 1 (0.005 mol) were taken in 100 ml RBF with 25 ml ethanol. To this reaction 2 gm of KOH was added & resulting reaction was stirred at room temperature for 24 hrs. Then contents were poured over crushed ice & acidified with conc. HCl, solid thus obtained were separated by filtration & crystallized from ethanol to get compound 3. Their characterization data is in the table-1(3a-3e).
Spectral data
3a I.R. (KBr, cm-1): 3059 (Ar =C-H),2920 (C-H ), 1649 (–C=O), 1581 ( –C=C), 1211 ( –C-F), 1022 (–C-Br); NMR (DMSO/ d6): δ 2.34 -3.36 (3H, s, CH3), 6.86-8.12 (6H, m, Ar & =CH protons ), 12.30 (1H, s, -OH).
Synthesis of Benzothiazepines
Compound 3 was dissolved in minimum quantity of ethanol. To this, 4-6 drops of 2-aminothiophenol was added and the resulting reaction was refluxed for 4 hrs. Then reaction mixture was acidified by using 2 ml acetic acid and heating was continued for next 4 hrs. After cooling pale yellow crystals 4 were obtained. These were filtered and purified by recrystallization from ethanol. The products obtained were identified with the help of spectral data. Their characterization data is given in the table 1(4a-4e).
Spectral data
4b:I.R. (KBr, cm-1):3448 (Ar- O-H), 1598 (C=N),1552 (C=C), 1207 (–C-F), 1042 ( –C-Br ); NMR (DMSO/ d6): δ 1.14 (1H, dd , C-H), 2.99 (1H, dd, C-H), 3.59 (1H, dd, C-H), 7.28-7.66 (8H, m, Ar-H), 7.90 (1H, d, Ar-H), 15.50 (1H, OH).
Synthesis of Pyrazoline
Compound 3 was taken in 100 ml RBF with 15 ml alcohol. To this reaction mixture 1 ml hydrazine hydrate was added & the contents were heated under reflux for 3 hrs and to this 2 ml acetic acid was added & heating was continued for further 2 hrs. After cooling contents were poured over crushed ice. The solid thus obtained was separated by filtration & crystallized with alcohol to get compounds 5. The products obtained were identified with the help of spectral data. Their characterization data is given in the table 1(5a-5e).
Spectral Data
5a: I.R.(KBr, cm-1) 3350 (N-H), 2987 (Ar- =C-H), 1602 ( –C=N), 1573 (–C=C), 1203 (C-F) 1047 ( C-Br); NMR(DMSO/ d6): δ 3.05 (1H, dd, C-H), 3.67 (1H, dd, C-H), 5.07 (1H, dd, C-H), 7.25-7.46 (6H, m, Ar and NH proton), 8.09 ( 1H, d, Ar-H) 11.82 (1H, OH).
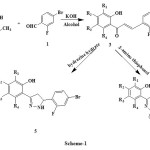 |
Scheme 1 Click here to View scheme |
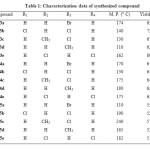 |
Table1: Characterization Data of Synthesized Compound Click here to View table |
References
- Komeilizadeh H., Iranian J. Pharma. Research, 2006, 4, 229.
- Ducki S., Forrest R., Hadfield J. A., Kendall A., Lawrence N. J., Mcgown A. T. and Rennison D. Bioorg. Med. Chem. Lett. 1998, 8, 1051.
- Parmar V. S., Sharma N. K., Husain M., Watterson A. C., Kumar Samuelson L. A., Ashok L. C., Prasad A. K., Kumar A., Jha H. N., Olesen C. E., Stove C. P., Bracke M. E. and Mareel, M. M. Bioorg. Med. Chem. 2003, 11, 913.
- Mukharjee S., Kumar V., Prasad A. K., Raj H. G., Bracke M. E., Olsen C. E., Jain S. C. and Parmar V. S. Bioorg. Med. Chem. 2001, 9, 337.
- Lopez S. N., Castelli M. V., Zacchino S. A., Dominguez J. N., Lobo G., Jaime C. C., Cortes J. C. G., Ribas J. C., Devia C., Ana M. R. and Ricardo D. E. Bioorg. Med. Chem. 2001, 9, 1999.
- Lin Y. M., Zhou Y., Favin M. T., Zhou L. M., Nie W. and Chen F. C. Bioorg. Med. Chem. 2002, 10, 2795.
- Nielsen S. F., Christensen S. B., Cruciani G., Kharazmi A. and Liljefors T. J. Med. Chem, 1998, 41, 4819.
- Li R., Kenyon G. L., Cohen F. E., Chen X., Gong B., Dominguez J. N., Davidson E., Kurzban G., Millar R. E., Nuzum E. O., Rosenthal P. J. and Mckerrow J. H. J. Med. Chem. 1995, 38, 5031.
- Liu M., Wilairat P. and Go M. L. J. Med. Chem. 2001, 44, 4443.
- Ko H. H., Tsao L. T., Yu K. L., Liu C. T., Wang J. P. and Lin C. N. Bioorg. Med. Chem. 2003, 11, 105.
- Matsuda H., Morikawa T., Ando S., Iwao T., and Masayuki Y. Bioorg. Med. Chem. 2003, 11, 1995.
- Herencia Ferrandiz M. L., Ubeda A., Dominguez J. N., Charris J. E., Lobo G. M. and Alcaraz M. J. Bioorg. Med. Chem. Lett. 1998, 8, 1169.
- Go M. L., Wu X. and Liu X. L. Curr. Med. Chem. 2005, 12, 483.
- Bhat A. R., Athar F. and Azam A. Eur. J. Med. Chem. 2009, 44, 926.
- Johnson M., Younglove B., Lee L., LeBlanc R., Holt H., Hills P., Mackay H., Bown T., Mooberry L. S. and Lee M. Bioorg. Med. Chem. 2007, 17, 5897.
- Carrion M. D., Luisa C., Lopez L. C., Camacho M. E., Tapias, V., Escames G., Castroviejo D. A., Espinosa A., Gallo M. A. and Entrena, A. Eur. J. Med. Chem. 2008, 43, 2579.
- Ramana M. V., Billa V. K., Pallela V. R., Murlidhar R. M. R., Boominathan R., Gabriel J. L. and Reddy E. P. Bioorg. Med. Chem. 2008, 16, 3907.
- Jiaxi X. Mol. Div. 2005, 9, 45.
- Levai A. J. Het. Chem. 1999, 37, 199.
- Urbankshi M. J., Chen R. H., Demarest K. T., Gunnet J., Look R., Ericson E., Murray W. V., Rybczynski P. J. and Zhang X. Bioorg. Med. Chem. Lett. 2003, 48, 4031.
- Nakayama K., Nozawa Y. and Fukuta Y., J. Cardiovasc. Pharmacol. 1994, 23, 731.
- Tarabova B., Lacinova L. and Engel J. Eur. J. Pharmcol. 2007, 753, 39.
- Santo R. D. and Costi R. II Farmaco 2005, 60, 385.
- Sondhi S. M., Kumar S., Kumar N. and Roy P. Med. Chem. Res. 2011, 1.
- Reddy M. V., Billa V. K., Pallela V. R., Mallireddigari M. R., Boominathan R., Gabriel J. L. and Reddy E. P., Bioorg. Med. Chem. Lett. 2008, 16, 3907.
- Sivakumar P. M., Prabhu Seenivasan S., Kumar V. and Doble M., Bioorg. Med. Chem. Lett. 2010, 20, 3169.
- Bandgar B. P., Gawande S. S., Bodade R. G., Gawande N. M. and Khobragade C. N. Bioorg. Med. Chem. 2009, 17, 8168.
- Nassar E. J. Am. Sci. 2010, 6.
- Al-Saadi Saudi M. S. M. Pharm. J. 2008, 16,135.
- Ahasan N. B. and Islam M. R. Bang. J. Phar. 2008, 2, 81.
- Rahman M. A. and Siddiqui A. A. Int. J. Pharm. Sci. Drug Res. 2010, 2.
- Bandgar B. P., Gawande S. S., Bodade R. G., Gawande N. M. and Khobragade C. N., Bioorg. Med. Chem. 2009, 17, 8168.
- Halnor V. B., Joshi N. S., Karale B. K. and Gill C. H., Indian J. Het. Chem.., 2005, 14, 371.
- Waheed V. and Khan S. A. Indian J. Het. Chem., 2002, 11, 59.
- Kimata A., Nakagawa H., Ohyama R., Fukuuchi T., Ohta T. Suzuki S. and Miyata N. J. Med. Chem. 2007, 50, 5053.

This work is licensed under a Creative Commons Attribution 4.0 International License.









