Research of nickel’s electrochemical property in sulfuric acid solution by using potentiodynamic polarization curve.
Bekenova Gulmira1, Baeshov Abduali, Yılmaz Satılmış
1Department of Social & Educational Sciences, SuleymanDemirel University, 040900, Kazakhstan
DOI : http://dx.doi.org/10.13005/ojc/310115
Article Received on :
Article Accepted on :
Article Published : 23 Feb 2015
In the presented work in order to deeply study the mechanism of electrode processes that take place while polarizing the nickel electrode in acid solutions by alternating current, potentiodynamic polarization curves were obtained.The detailed study of shapes of polarization curves; their dependence on concentration, temperature, and other physical and chemical parameters, gives opportunity to obtain full information on the nature and kinesthetic of processes taking place on electrode surface. The electrochemical properties of nickel electrode were researched by estimating cyclic potentiodynamic polarization curves in sulfuric acid medium; and the influence of electrolyte concentration, potential giving speed, the temperature of solution on anodic and cathode processes were also studied. The meanings of transfer number (an) and diffusion (D) coefficient, the reaction order of metal ions during the process of nickel electrode’s anode corrosion in sulfuric acid solution, and the activation energy is estimated. Result of the calculations showed that nickel’s melting process goes in mixed, diffusion-kinetic regime. It was found that the raising of solution’s temperature increases the height of corrosion current. The results of the experiments done during the application of cyclic polarization curves showed that the electrochemical processes that take place in polarization by industrial alternating current in anode and cathode half periods is different from those done in stable current and with more complex mechanism.
KEYWORDS:Nickel electrode; Potentiodynamic polarization curves; Alternating current; Electrodeposition
Download this article as:| Copy the following to cite this article: Gulmira B, Abduali B, Satılmış Y. Research of nickel’s electrochemical property in sulfuric acid solution by using potentiodynamic polarization curve. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Gulmira B, Abduali B, Satılmış Y. Research of nickel’s electrochemical property in sulfuric acid solution by using potentiodynamic polarization curve. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7353 |
Introduction
Nowadays nonferrous metal industry is interested in using hydrometallurgical processes which are more cost-effective and ecologically more safe than pyrometallurgical ones [1-2]. The demand for nickel and its alloysare being increasing to use in everyday life and industrial processes widely and rapidly. By studying the ways of getting needed compounds by melting nickel in metal type or solid wastes containing nickel, we can not only increase the resources of this metal but also decrease the production cost of its components. [3-4-5] studies show that passivation liable nickel’s melting is accelerated by industrial alternating current polarizing in sulfuric acid medium.
In [6] study it is presented that in nickel electrode 0,5 М H2SO4 solution in anode polarization curvestransmitted in potential giving speed of 5 mV/s, in “plus”1,4-1,7 V potential range the metal passivized and 4-6 nm thickness oxide layer with electron passing property is developed .
In these scholarly works the possibility of occurrence of the electrochemical reactions on the oxide surface, that is the development of oxygenic ion radicals as the intermediate product when aqueous molecules corrosion occurs in potential’s high anode areas (fields) is stated.
Oxygen ion radical –ОН in active centers, that is by transferring gradually its charge to metal ions in oxide layer’s crystal gridit undergoes adsorption, and as a result develops complex Ni[NiO(OH)] (1st reaction).
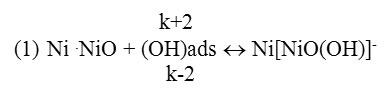
According to 2nd reaction it decomposes, and undergoes the desorbtion by transferring Ni2+ions to the solution.
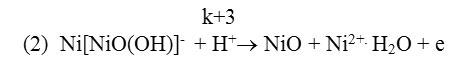
Koytun et al.[6]presents that on anode polarization curve put on nickel electrode in sulfuric media, when potential shifts to positive value, the metal firstly melts according to the regularity; in high potentials it passivates, passivating products (oxide layers) cover fully the surface of the electrode and completely stops metal’s melting.The above mentioned studies prove that not all findings and conclusions are presented on electrochemical properties of nickel electrode. Thus it is important to study the electrochemical properties of nickel polarized by alternating current.
Experimental Method
The volt-ampere method that describes the connection between electric current density and electrode potential was selected as the main method to research electrochemical reactions’ kinesthetic that occur while melting nickel with alternating current. The polarization curves were obtained by estimating various cyclic potentiodynamic curves in SVA – 1 BM potentiostat. The main polarization curves were estimated at potential linear change speed of 10 mV/s,and the polarograms were obtained by Н307/1 two-dimensional tablet potentiometer. Argentum chlorine electrode (Е=+0,203 V) was selected as comparative electrode and platonic electrode as auxiliary one. The polarization curve was estimated on the nickel electrode surface with 0.02 cm2 in area. Before estimating polarization curves each time the electrodes were grinded by flint paper, washed by distilled water and wiped by filter paper.
Results of Research
In the presented work the mechanism of electrode processes that take place while polarizing the nickel electrode in acid solutions by alternating current, potentiodynamic polarization curves were obtained.
In order to understand mechanism of processes that occur in nickel electrodes when they are polarized by industrial frequency alternating current, anode-cathode cyclic potentiodynamic polarization curves were measured (Figure 1).
On cathode-anode cyclic potentiodynamic polarization curves derived from sulfuric acid solution with 1 M concentration, only the hydrogen release current was noticed at cathode polarization direction «minus» 0,5 V potential area (Figure 1, curve 2).
When potential started to shift to the direction of reverse anode in about 0.25 V the maximum of the corrosion current was measured according to 3rd reaction.
Ni – 2e →Ni2+ E0=-0.250 B(3)
Positivelypotential ranges (1,50V -1,65 V) the second wave of corrosion was stated. This corroding current can be predicted as due to 4-8 reactions resulting in development of nickel oxides [7]:
(4) Ni + H2O = NiO + 2H+ + 2e–E0=0.11 6B
(5) Ni2O3 + 2H+ + 2e = 2NiO + H2OЕ0=1,032 В
(6) 2Ni3O4 + H2O = 3Ni2O3 + 2H+ + 2e– E0=1,305 B
(7) Ni2O3 + H2O = 2NiO2 + 2H+ + 2e– E0=1,434 B
(8) Ni2+ + H2O = NiO2 + 4H+ + 2e– E0=1,593 B
In order to define this hypothesis the electron-microscopic analysis was conducted by the help of EM-125 K microscope on layer of oxide developed on nickel electrode surface that had been anodic polarized.
The analysis of the surface of nickel electrode showed the development of two and three valence nickel oxides, the selected area diffraction patterns are presented in 2-picture. Then when the electrode potential reached «plus» 1,7 -1,8V meanings the oxygen release current was stated.
When potential is directed from anode to cathode, the wave of re-corrosion of high valence oxide, which was developed on the surface of electrode, can be seen.
These phenomena present that mechanism of electrochemical reactions is more complex when electrodes are polarized by alternating current.
In cyclic anode-cathode potentiodynamic polarization curve (Figure 1, curve 1) the corrosion reactions in the main nickel electrode are repeated, but it is seen that when potential moves from anode to cathode the hydrogen release potential shifts to reverse area.
In this case the increase of hydrogen intensity can be explained by the change of the structure of the nickel electrode’s surface. This phenomenon shows that there is metal oxide left on the surface of the nickel electrode.
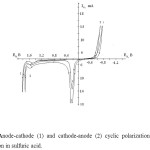 |
Figure1: Anode-cathode (1) and cathode-anode (2) cyclic polarization curves of nickel electrode’s solution in sulfuric acid. |
According the results of the research it was found that the speed of nickel melting depends on the concentration of sulfuric acid. If the concentration is increased the maximum current of nickel’s melting is at the first increased ( till 1,0 mol/L), then it decreases (till 5,0 mol/L) (Figure 3). At this moment, it can be certain that the black colored oxide layer is developed on the surface of the electrode. On polarogram it can be seen that with the increase of the concentration of sulfuric acid the potential meaning of the second wave of nickel’s corrosion (the development of nickel oxide) shifts to the reverse side. It is well known from the literature review that the increase of the concentration of sulfuric acid easies the development of metal oxides on the anode surface [8]. Along with this it is also known that in aquatic solutions,when the concentration increased, the own electrical conductivity of the acids at first raises, reaches maximum meaning and then it decreases [9]. Like in other acids [10], in defined concentration sulfuric acid hydrogen ions reach their maximum activity.
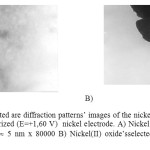 |
Figure2: The selected are diffraction patterns’ images of the nickel oxides developed on the surface of the anodic polarized (Е=+1,60 V) nickel electrode. A) Nickel (ІІІ) oxide’sselected area diffraction pattern image » 5 nm х 80000 B) Nickel(ІІ) oxide’s selected area diffraction pattern image » 5 nm х 80000. Click here to View figure |
According to the information from the experiment, given the sulfuric acid concentration is not high, the reaction that was estimated from the dependence given at lg[H2SO4]-lgi, was approximately 1.03.
In given dependence, in high concentration of H2SO4 the linear connection has sudden drop, this shows that the nickel’s melting mechanism is sharply changed due to the acid’s concentration.
In sulfuric acid medium, by changing the speed of giving the potential in the range of 5-100 mV/s nickel electrode, the metal anodic corrosion potentiodynamic polarization curves were estimated (figure 4.).When potential giving speed was increased the maximum of the nickel’s first corrosion current (nickel ions’ development current) firstly (5-20 mV/s) proportionally increased and then later the small increase was seen.
The increase in the relationship between the potential giving speed and the limited current amount shows that nickel’s melting process goes in mixed, diffusion-kinetic regime.
By processing the polarization curve by Galus method [11] the transference number of nickel ions (αn) and diffusion (D) coefficient was defined.
For electrochemical polarization in potential giving’s linear connection, the polarization curve is described using the equation of Rendels–Shevchik:
I=αn FAC0Db(bt), here b=αnFv/RT (9)
Here, А-electrode area;αn–transference number; D- diffusion coefficient of depolarization, cm2/s; v-speed of potential giving, V/s; RT – fixed gas and temperature according to Kelvin scale, t-time, (bt)-potential function.
250С current’s height equation described by the following dependence:
I=3,0. 105×αn (αn)1/2× AD1/2×v1/2× C0 (10)
10 – as it can be seen from the equation, the current’s height is proportional to the square root of diffusion coefficient and potential giving spped.
Nicolson and Shain theoretically calculated (bt) function’s dependence on potential (defines current’s height by putting potential meaning) gives possibility to define wave’s potential (Еn) according to the giving speed [12]:
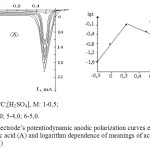 |
Figure3: Nickel electrode’s potentiodynamic anodic polarization curves estimated in different concentrations of sulfuric acid (A) and logarithm dependence of meanings of acid concentration and limited current height (B) Click here to View figure |
![Figure4. Nickel electrode’s potentiodynamic anodic polarization curves estimated in different speeds of potential giving, [H2SO4]=1 M; t=200C; v= 1-5; 2-10; 3-20; 4-50; 5-100 mB/S; [H2SO4]=1 M; t=200C.](http://www.orientjchem.org/wp-content/uploads/2015/02/Vol31_No1_Rese_Beken_Fig4-150x150.jpg) |
Figure4: Nickel electrode’s potentiodynamic anodic polarization curves estimated in different speeds of potential giving, [H2SO4]=1 M; t=200C; v= 1-5; 2-10; 3-20; 4-50; 5-100 mB/S; [H2SO4]=1 M; t=200C. Click here to View figure |
En=E0 – R [0,78 – lnK + lnDb] / αn F = P – RTlnv / 2αn F = a + b ln v (11)
That is it is proportional to logarithm of the potential giving speed. So the above given equations help to process polarization curves, and gives possibility to define transference number.of nickel ions (an) and diffusion coefficient (D).
E-lnv equation and the «а» and «b» coefficients are defined by using the method of the least squares, and the later here is equal to RT/(2αnF). From this meaning the αn is estimated. By putting the meaning of αn from the angular coefficient to 11-equation the diffusion coefficient meaning is defined.
 |
Figure5: Potentiodynamic polarization curvesat different temperatures of the solution (A) and the logarithms of the solution temperature and height of the oxidation current (B) Click here to View figure |
Overall, the diffusion coefficient (D) meanings of nickel ions’ estimated transference number.(αn) are as following: (αn)=1,4×10-2, D=1,039×10-2 cm2/s.
In many cases, the definition of the impact of the temperature on reaction speed in the process of electrochemical reactions is – the research on the impact of temperature on the current density in the process of the stable polarization, thus the straight linear image in lgi –l/T coordinates is noticed in concentration and chemical polarization. The activation energy of electrochemical reaction is found according to the meaning of angular coefficient of straight linear pattern.
In our study the influence of the temperature on the corrosion of nickel to nickel’s ions is also discussed (Figure 5). Here the height of the limited current is raised if the solution temperature is increased. The meaning of activation energy estimated by applying the temperature-kinetic method is as following: for nickel’s corrosion of 1-ΔЕ1=0,15 V is 14,6 kJ/mol, for 2 – ΔЕ2=0,175 V is 18,4 kJ/mol, for 3 – ΔЕ3=0,20 Vis 23,5 kJ/mol, for 4 – ΔЕ4=0,25 Vis – 27,1 kJ/mol, and the average meaning is 20,9 kJ/mole. This shows that the undergoing electrochemical reactions are happening in diffusion-kinetic regime.
Conclusion
To sum up, the study discussed the impact of sulfuric acid concentration, the potential giving speed and the solution temperature on melting process of nickel by applying potentiodynamic polarization curves.
The meanings of transference number.(an) and diffusion (D) coefficient, the reaction order of metal ions during the process of nickel electrode’s anode corrosion in sulfuric acid solution, and the activation energy is estimated. It was found that the raising of solution’s temperature increases the height of corrosion current.
The results of the experiments done during the application of cyclic polarization curves showed that the electrochemical processes that take place in polarization by industrial alternating current in anode and cathode half periods is different from those done in stable current.
References
- Shangyu Wang, (1997) Electrochemical Properties of Nanocrystalline Nickel and Nickel-Molybdenum Alloys. Queen’s University Kingston, Ontario, Canada.
- Kimberly M. Lincoln , Michael E. Offutt , Travis D. Hayden , Ryker E. Saunders , and Kayla N. GreenStructural, Spectral, and Electrochemical Properties of Nickel(II), Copper(II), and Zinc(II) Complexes Containing 12-Membered Pyridine- and Pyridol-Based Tetra-azaMacrocycles. Department of Chemistry, Texas Christian University, Fort Worth, Texas 76109, United States Inorg. Chem. .,2014, , 53 (3): 1406–1416.
- Bekenova G.S.,(2007)Electrochemical properties of nickel electrode polarized by alternating current in aquatic solutions.Dissertation, Almaty.
- Belov S.F., Brukvin V.A., Levin A.V., Kuznetsova O.G The study of metal nickel solubilization under influence of the industrial frequency alternating current in sulfuric acid electrolytes,non-ferrous metals, ., 2005.,17:39-41.
- Bekenova G.S., Baeshov A.B., Konyrbayev A.E. Electrochemical properties of nickel electrode polarized by alternating current in sulfuric acid solution // Science and Education of South Kazakhstan, Chemistry, chemical technology series,Shymkent, 2004., 35:17-21.
- Kovtun V.N., Bolotin A.VDynamic behavior of Ni-H2 SO4 system at high anodic potentials and different electrolysis conditions, Electrochemistry .2005.,41(1):111-115.
- Nikolsky B.P. (Ed.)Handbook of chemistry: Leningrad: Chemistry, 1964, V.3, 2nd edition.
- FedioteffN.PApplied chemistry, Leningrad: Chemistry, .1962 pp.355-358.
- Antropov A.I. Theoretical electrochemical,Moscow: High school, 1984., p.519.
- Levin A.I. Theoretical foundations of the electrochemistr, Moscow:Metallurgizdat, 1963.,p.430.
- Galus Z Fundamentals of Electrochemical Analysis,Moscow: Mir, ,1974 p.552.
- Nicolson K.S., Shain ICalculation of chomovoltamperemetris measuring, Anal. Chem. .1964.,V.36., p:706-708

This work is licensed under a Creative Commons Attribution 4.0 International License.









