Removal of hexavalent chromium ions from aqueous solution by adsorption using activated carbon prepared from Cucumis Melo Peel Activated carbon
M. Manjuladevi1 and S. Manonmani2
1Department of Chemistry, SNS College of Technology, Coimbatore 641035, India
2Department of Chemistry, PSG College of Arts and Science, Coimbatore, India
DOI : http://dx.doi.org/10.13005/ojc/310166
Article Received on :
Article Accepted on :
Article Published : 21 Mar 2015
Activated carbon prepared from Cucumis Melo Peel was used as adsorbent for the removal of chromium ion from aqueous solution. Adsorption experiments were performed by varying initial metal ion concentration, temperature, and pH. Freundlich and Langmuir isotherms were used to analyze the equilibrium data obtained at different adsorption conditions. Adsorption results obtained, shows that the Cr (VI) uptake being attained at PH 3. The equilibrium adsorption data was better fitted to the Langmuir’s and Freundlich adsorption models.. The result indicates that the Cucumis Melo Peel could be used to effectively adsorb Cr (VI) from aqueous solution.
KEYWORDS:Removal; Activated carbon; Heavy metal; Cucumis Melo Peel
Download this article as:| Copy the following to cite this article: Manjuladevi M, Manonmani S. Removal of hexavalent chromium ions from aqueous solution by adsorption using activated carbon prepared from Cucumis Melo Peel Activated carbon. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Manjuladevi M, Manonmani S. Removal of hexavalent chromium ions from aqueous solution by adsorption using activated carbon prepared from Cucumis Melo Peel Activated carbon. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7941 |
Introduction
Heavy metals are major pollutants in marine; ground and industrials, and even in treated wastewater. The presence of these metals in the environment has been a great concern because of their toxic nature and other adverse effects on receiving waters. Among these heavy metals are chromium, copper and zinc, and ingestion beyond the permissible quantities can cause various chronic disorders in human beings. It is well known that heavy metals can damage/harm nerves, liver and bones, and they also block functional groups of essential enzymes [1]. Also, acute systemic poisoning can result from high exposure to hexavalent chromium [2]. Most of the chromium ions in wastewater, especially hexavalent chromium originates from industries such as electroplating, metal finishing and magnetic tape manufacturing. For these industrial groups, chromium is a problematic one. Tanning is one of the oldest and fastest growing industries in India. Chromium, which is on the top priority list of toxic pollutants defined by the US Environmental Protection Agency (EPA) exists in nature mainly in two oxidation states +3 and +6. It is a bioelement in the +3 state but mutagenic in the +6 state. Therefore, the speciation of chromium in contaminated environment becomes critical for understanding its fate and exposure. The hydrolysis behavior of Cr (III) is complicated and it produces mononuclear species Cr(OH)2+, Cr(OH)2+, Cr(OH)4- and Cr(OH)30, the polynuclear species Cr2(OH)2 and neutral species Cr3(OH)40. The hydrolysis of Cr6+ produces only neutral and anionic species. At pH greater then 6.5, Cr (VI) exists in the form of CrO42-. Chromium (VI) compounds are found to be more toxic than Cr(III) compounds because of their high solubility in water and consequently high mobility. The drinking water guideline recommended by the US EPA is 100 μg/L Cr(VI). Several metal ion removal techniques have been targeted as possible solutions. Ion exchange[3], reduction[4], chemical precipitation[5], polymer based membrane separation[6, 7], adsorption[8], electrochemical precipitation[9], solvent extraction[10], cementation[11] and electro kinetic remediation[12] are among the available methods to accomplish the reduction of metal concentration. Nevertheless many of these approaches are marginally cost effective or difficult to implement in developing countries. Therefore, the need arises for a treatment strategy that is simple and robust, and also addresses local resources availability and constraints. Adsorption is an effective and that versatile method for removing chromium particularly when combined with appropriate regeneration steps. This solves the problems of sludge disposal and renders the system more economically viable especially if low cost adsorbents are used. Varieties of activated carbons are commercially available but very few of them are selective for heavy metals and most of them are very costly/expensive[13]. Despite the prolific use of activated carbon [14], in wastewater treatment the price of this material is quite expensive and there is a definite need for substitute materials to suit these demanding applications. The solid materials should be able to be regenerated with simultaneous quantitative recovery. For the past few years
Materials and Methods
Adsorbent
Cucumis Melo Peel was collected from in and around pazhamudir nilayam of Coimbatore. The collected peels were cut into small pieces, washed with tap water several times to remove dust and dirt rinsed with deionised distilled water and then dried. The dried musk melon.
Adsorbate Solution
All the Chemicals used to prepare reagent solution were of analytical reagent grade. The stock solution to obtain 1000 mg Cr(VI) solution was prepared by dissolving K2Cr2O7 in double distilled water. The solution was diluted to the desired concentration (from 100 mg/L to 400 mg/L) for experiment. Before the batch mode experiments were carried out, the pH of the solution was adjusted to the required value for the adsorption of Cr(VI) ion (which was 3 according to previous batch study by there has been developing interest in the preparation of low cost adsorbents as alternatives to activated carbon in water and wastewater treatment processes. In several previous reports, many investigators have studied the feasibility of less expensive materials such as alginate beads[15], wheat straw[16], carbon develop from waste material[17], biosorbents[18], activated sludge[19], fly ash[20] and agricultural waste[21], for the removal of chromium from waste water. However the problems associated with these adsorbents are the regeneration and recovery processes, which made them unattractive for wider commercial applications. This calls for a research effort to develop an industrially viable, cost effective and environmentally compatible technology for the removal of chromium from wastewater. In this study, we have derived a low cost activated carbon from agricultural waste, namely Cucumis Melo Peel (CMAC) for the removal of hexavalent chromium from synthetic waste water. Cucumis Melo Tree is largely populated tree in Persia.
peels were placed in the muffle furnace and carbonization was carried out at 200oC for 2 hrs. The carbonized material was ground to a fine powder. The resulting material was sieved in the size range of 125-250 mesh particle size. It was placed in an air tight container for further use.
adding 0.1 N sodium hydroxide (or) /or 0.1 N hydrochloric acid.
Stock solution of Cr(VI) was prepared by dissolving appropriate amount of K2Cr2O7 in distilled water. This solution was diluted to obtain standard solution containing 100-400 mg/L Known amounts of CMAC was added and solution pH was adjusted using dilute hydrochloric acid or sodium hydroxide. The solution was agitated at 750 rpm in orbital shaker at 30 ºC. The solutions were centrifuged and the amount of Cr(VI) in the supernatant was measured by using atomic absorption spectrometer.
Adsorption (%) = — x 100
CI
Effect of pH
Adsorption isotherms were carried out with different initial concentrations of Cr(VI), while the carbon dosage was fixed for the effect of pH. Control experiments were also carried out without adsorbents. The percentage of Cr(VI) adsorption from aqueous solution was computed by the following equation:
CI – CF
Result and Discussion
pH is one of the most important parameter controlling the metal ion adsorption process. The effect pH on the adsorption of Cr(VI) is attributed to interaction between ions in solution and complex formed at the adsorbent surface. The fact that Cr(VI) in aqueous solution can form different species in the solution at various pH ranges. This study was carried out at initial Cr(VI) concentration of 100 mg/l. The adsorption of Cr(VI) on the prepared adsorbents decreased with increasing pH. Table 1 show that CMAC is active in acidic range especially at low pH range. The maximum adsorption of Cr (VI) species on the two different adsorbents was found to be optimum at pH 3.0 and negligible at pH over 9.0. Cr(VI) can exist in several stable forms such as CrO42-, HCrO42-, Cr2O72- and HCr2O7– and the relative abundance of a particular complex depends on the concentration of chromium ion and pH of the solution. At low pH, the sorbent is positively charged because of the protonation, where as the sorbate like dichromate ions exists mostly as an anion, leading to an electrostatic attraction between the sorbent and the sorbate. This results in increased adsorption at low pH. As the pH of the solution increases, the sorbent undergoes deprotonation and the adsorption capacity decreases. Therefore all subsequent studies were carried out at pH 3.0. The high removal of Cr(VI) at low pH was reported earlier using different adsorbents [17, 20].
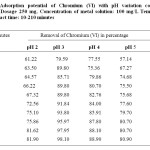 |
Table1: Adsorption potential of Chromium (VI) with pH variation conditions: Adsorbent Dosage 250 mg, Concentration of metal solution: 100 mg/L Temperature 30oC, Contact time: 10-210 minutes Click here to View table |
Effect of Contact time
The effect of contact time on the removal of Cr(VI) was studied in the pH range 3.0 for CMAC. The amount of adsorbent was fixed 100 mg/l and the solution was equilibrated. An aliquot of the solution was periodically with drawn at an interval of one hour and analyzed using spectrophotometer to establish the Cr(VI) removal. The results are shown in Table 2. The percent of removal increases from 79.59 to 98.10 with time (10 to 210 minutes) and attains equilibrium at 3 hrs. It is clear that, at the beginning, % removal increased rapidly in few minutes, by increasing contact time, removal increased lightly and slowly till reach maximum value and this can be explained on the basis that as initially a large number of vacant surface sites are available for adsorption of metal ions but with passage of time the surface sites become exhausted (Zhan et al, 2000). These results indicate that the activated carbon has a very strong capacity for adsorption of Cr(VI) ions in solutions
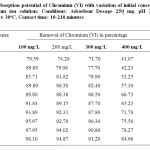 |
Table2: Adsorption potential of Chromium (VI) with variation of initial concentration of Chromium ion solution: Conditions: Adsorbent Dosage 250 mg, pH 3+ 0.02, Temperature 30oC, Contact time: 10-210 minutes Click here to View table |
Effect of carbon dosage
The experiments were conducted to find out the minimum amount of carbons required for the removal of Cr(VI). It is evident that for the quantitative removal of 100 mg/L of Cr(VI) in 100 ml reference solution, a minimum dosage of 250 mg is required for 98.1 % removal in the case of CMAC. The effect of adsorbent dose on Cr(VI) uptake was investigated by varying the adsorbent dose of CMAC from 50 to 250 mg. The initial concentration of metal solution used in this study was 100 mg/L and the experiments were carried out at pH 3.0 by varying the contact time from 10 to 210 minutes at 30oC. Experimental results showed that the percentage removal Cr(VI) increases from 26.55% to 98.10% with the increasing amount of adsorbent dosage (Table 3). This may be due to the increase in effective surface area and increasing availability of active adsorption sites from the increase in adsorbent dosages.
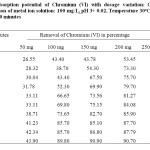 |
Table3: Adsorption potential of Chromium (VI) with dosage variation: Condition: Concentration of metal ion solution: 100 mg/L, pH 3+ 0.02, Temperature 30oC, Contact time: 10-210 minutes Click here to View table |
Kinetic of Adsorption
Adsorption kinetic provides valuable information about the reaction pathways and mechanism of the reactions. The pseudo-first order equation is applied to model the kinetics of Chromium (VI) adsorption on to activated CM peel.
Pseudo-first order model
Lagergren proposed a Pseudo-first order kinetic model. The integral form of the model is
log (qe-q) = log qe – (Kad/2.303)t
where q is the amount of Cr(VI) adsorbed (mg/g) at time t (min). qe is the amount of Cr(VI) adsorbed at equilibrium (mg/g), and Kad is the equilibrium rate constant of pseudo-first-order adsorption (min-1). This model was successfully applied to describe the kinetics of many adsorption systems.
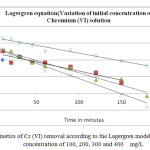 |
Figure1: Kinetics of Cr (VI) removal according to the Lagergren model at initial feed concentration of 100, 200, 300 and 400 mg/L
|
The applicability of the above model can be examined by linear plot of log (qe-q0) versus t, and is presented in Fig.1. To quantify the applicability of above model, the correlation coefficient (R2) was calculated from the plot. The linearity of this plot indicates the applicability of the above model. However, the correlation coefficient (R2), showed that the pseudo-first order model, an indication of a chemisorptions mechanism, fits better the experimental data (R2 is in the range of 0.94-0.98).
Adsorption Isotherms
The equilibrium of adsorption is one of the most important physico-chemical aspects for the evaluation of the process, as the distribution of Cr(VI) between the liquid phase and the solid adsorbent phase is a measure of the position of equilibrium in the sorption process and thereby is critical in optimizing the use of adsorbent. Expressed as a mathematical model, adsorption isotherms are also of great importance for they provide vital inputs in the description of how adsorbate interacts with an adsorbent. As a requisite, in the present investigation the kinetics of the adsorption data was analyzed, with the conformity between experimental data and the model predicted values being expressed by the correlation coefficient (R2). A relatively high R2 value indicates successful applicability of the model in describing the kinetics of the adsorption (Krim Louhab, 2010). For the current research, the results of the concentration dependence of chromium adsorption on activated carbon over initial chromium ion concentrations 10-100 mg/L were fitted to the 3 equilibrium models namely the Langmuir, Freundlich , Tempkin and Dubinin-Radushkevich (D-R). These were applied to evaluate feasibility of adsorbate-adsorbent interaction and subsequently adsorbent interaction and subsequently analyzed for validity of the study (22),
Langmuir adsorption isotherm
Langmuir adsorption isotherm is based on the assumption that points of valency exist on the surface of the adsorbent and that each of these sites is capable of adsorbing only one molecule. Thus the adsorbed layer will be one molecule thickness. Further, it is assumed that all the adsorption sites have equal affinities for the adsorbate and the presence of adsorbed molecules will not affect the adsorption of molecules at an adjacent site.
The Langmuir adsorption isotherm is commonly given by
x/m = k1Co/1 + k1Ce
where, x is the amount of chromium metal ion adsorbed (mg/L), m is the weight of adsorbent (mg), Ce is the concentration of metal solution at equilibrium and k1 is a Langmuir constant corresponding to the measure of maximum energy of adsorption capacity. On rearranging, the above equation becomes,
m/x = 1/ (k1/ k11) + 1/ k1Ce
A linear plot of 1/ Ce versus m/x (fig1, 2) shows the applicability of Langmuir adsorption isotherm (10) for the present study indicating the formation of monolayer coverage of adsorbate on the surface of the CMAC used in this study.
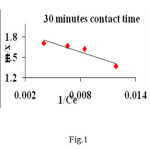 |
Figure1A: Langmuir isotherms plot for adsorption of heavy metal, Cr(VI) on adsorbent Click here to View figure |
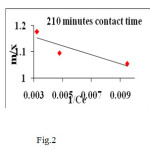 |
Figure 2 Click here to View figure |
Separation factor (RL): The essential characteristics of Langmuir isotherm can be expressed in terms of a dimensionless constant, separation factor or equilibrium parameter ‘RL’ which is defined by RL = 1/1 + bCi where Ci is the initial concentration of the metal solution and b is the Langmuir constant (k1).
The value of RL and the feasibility (23, 24) of the adsorption process is as follows:
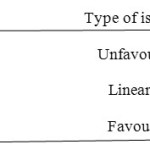 |
Table4 A Click here to View table |
In this study, RL values obtained are in the range 0.306-0.099 (below 1) indicates the feasibility of the adsorption of metal ion from aqueous solution with the adsorbent CMAC (Table 4) and also suggests the homogeneous distribution of active sites on the dried CMAC surface.
Freudlich adsorption isotherm
Freundlich isotherm is given by the equation:
Log x/m = logKf + 1/nlogCe
Where Kf and 1/n are Freundlich constants, which are related to the adsorption capacity and adsorption intensity respectively and x/m is the amount of adsorbate in an aqueous solution at equilibrium. The Freundlich adsorption isotherm plots at 32oC are obtained by plotting log x/m versus log Ce (Fig 3, 4) for different concentrations of metal ion solutions.
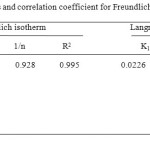 |
Table4B: The values of parameters and correlation coefficient for Freundlich and Langmuir isotherm model Click here to View table |
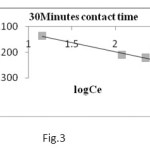 |
Figure 3 Click here to View figure |
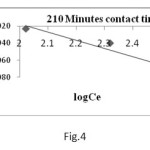 |
Figure 4 Click here to View figure |
The plots obtained are linear showing the applicability of Freundlich adsorption isotherm for the removal of Chromium metal. The values 1/n ranges from 0 to 1 (0.928) confirms the favourable adsorption of chromium metal onto CMAC. The correlation coefficient (R2 = 0.94) shows the good mathematical fit of Freundlich adsorption isotherm with the experimental data.
Dubinin-Raduskevich (D-R) isotherm
The D-R isotherm is more general because it does not assume a homogenous surface or constant adsorption potential [25]. It was applied to estimate the porosity apparent free energy and the characteristics of adsorption. The linear form can be represented as
Lnqe = lnqD – Bε²
where,
B is a constant related to the mean free energy of adsorption (mol2 (kJ2)-1)
qD is the theoretical saturation capacity (mg/g)
ε is the polyani potential, and calculated as follows:
ε = RTln (1+1/Ce)
The slope of the plot of ln qe versus ε² gives B and the intercept yields the adsorption capacity, qD.
Fig.5 shows D-R plot and the results are given in Table 5. The mean free energy of adsorption (E) (KJmol-1) is calculated from the equation
E= 1 / (2B) 0.5
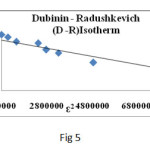 |
Figure 5 Click here to View figure |
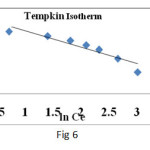 |
Figure 6 Click here to View figure |
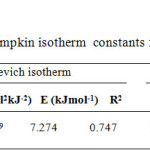 |
Table5: Dubinin Radushkevich and Tempkin isotherm constants for Cr(VI) adsorption on MMPAC Click here to View table |
Tempkin isotherm
The derivation in Tempkin isotherm assumes that fall in the heat of adsorption is linear rather than logarithmic,
as implied in Freundlich equation [26]. The Tempkin isotherm is applied in the following form
qe= RT/b (ln(ACe)
The linear form of Tempkin equation is
qe = βlnα + βlnCe (7)
Where,
β = (RT)/b,
T is the absolute temperature in Kelvin
R is the Universal gas constant, 8.314 J (mol K)-1 b is the Tempkin constant related to heat of sorption (J/mg) A the equilibrium constant corresponding to the maximum binding energy (L/g). The Tempkin constants α and b are calculated from the slope and intercept of qe versus lnCe (Fig. 6) and parameters are given in the Table 5.
Conclusions
The results of this investigation show that activated carbon developed from Cucumis Melo peel has a suitable adsorption capacity for the removal of Chromium metal ion from aqueous solutions. The experimental results were analyzed by using Langmuir, Freundlich, Tempkin and Dubinin-Radushkevich isotherm models and the correlation coefficients for Langmuir, Freundlich and Tempkin equations fitted better than Dubinin-Radushkevich equations. The kinetics study was performed based on pseudo-first order equation. The maximum removal of chromium metal was found to be 98.10% at pH 3 with 250 mg of the adsorbent when the concentration of the metal solution used was 100 mg/L. Based on the above investigations, the present study suggests that CMAC could be employed as a promising low-cost adsorbent for the removal of Chromium metal ions from aqueous solution.
References
- Nourbakhsh, M.N.; Kilicarslan, S.; Ilhan, S.; Ozdag, H. Sp. Chem. Eng. J., 2002, 85, 35.
- Ulmanu, M.; Anger, I.; Lakatos, J.; Aurn, G. in Proceeding of the First International Conference on Environmental Research and Assessment, Bucharest, Romania, March 23-27, 2003, 185-192.
- Rengaraj, S.; Joo, C.K.; Kim, Y.; Yi, J. J. Haz. Mater. 2003, 102, 257.
- Seaman, J.C.; Bertsch, P.M.; Schwallie, L. Environ. Sci. Technol., 1999, 33, 938,
- Zhou, X.; Korenaga, T.; Takahashi, T.; Moriwake, T.; Shinoda, S. Water Res. 1993, 27, 1049.
- Kozlowski, C.A.; Walkowiak, W. Water Res. 2002, 36, 4870.
- Chakravarti, K.A.; Chowdhury, S.B.; Chakrabarty, S.; Chakrabarty, T.; Mukherjee, D.C.A. Physicochem. Eng. Aspects 1995, 103, 59.
- Dahbi, S.; Azzi, M.; Guardia, M. Fresenius J. Anal. Chem. 1999, 363, 404.
- Kongsricharoern, N.; Polprasert, C. Water Sci. Technol. 1996, 34, 109.
- Pagilla, K.; Canter, L.W. J. Environ. Engg. 1999, 125, 243.
- Lin, C.F.; Rou, W.; LO, K.S. Water Sci. Technol. 1992, 26, 2301.
- Sawada, A.; Mori, K.; Tanaka, S.; Fukushima, M.; Tatsumi, K. Waste Management 2004, 24, 483.
- Mohan, D.; Sing, K.P.; Sing, V.K. Ind. Eng. Chem. Res. 2005, 44, 1027.
- Lalvani, S.B.; Wiltowski, T.; Hubner, A.; Wetson, A.; Mandich, N. Carbon 1998, 36, 1219.
- Ibanez, J.P.; Umestsu, Y. Hydrometallurgy 2004, 72, 327.
- Chun, Li.; Hongzhang, C.; Zuohu, L. Biochem. 2004, 39, 54.
- Selomulya, C.; Meeyoo, V.; Amal, R. J. Chem. Technol. Biotechnol. 1999, 74, 111.
- Gupta, V.K.; Shrivastava, A.K.; Jain, N. Water Res. 2001, 35, 4079.
- Aksu, Z.; Acikel, U.; Kabasakal, E.; Tezer, S. Water Res. 2002, 36, 3063.
- Gupta, V.K.; Park, K.T.; Sharma, S.; Mohan, D. Environmentalist 1999, 19, 129.
- Babel, S; Kurniawan, T.A. Chemosphere. 2004, 54, 951.
- Rajesh Gopinath,and Nandini Venugopal,Ultra Chemistry 2012, 333-340 .
- Periasamy, K.; Namasivayam, C. Waste Management 1995, 15, 63.
- McKay, G.; Blair, S.; Garden, J.R. Appl. Polym. Sci. 1982, 27, 3043.
- Vikal Guptha; Jaya Agarwal; Manisha Purohit; Veena.(2007), Res. J. Chem. Environ., 1991, pp. 40-43
- Necip Ata; Asim Olgun. (2009): Desalination, 249, pp.109-115.

This work is licensed under a Creative Commons Attribution 4.0 International License.









