One-pot green and efficient synthesis of xanthenedione derivativesusing [C4 (mim) 2](FeCl4)2as a magnetic room temperature dicationic ionic liquid
Bijan Mombaini-Goodajdar,1*Ali Reza Kiasat2, Ali Ezabadi3
1Department of Chemistry, Islamic Azad University Omidiyeh Branch, Omidiyeh, Iran
2Department of Chemistry, College of Science, Shahid Chamran University, Ahwaz, Iran
3Department of chemistry, Faculty of Science,Islamic Azad University, Central Tehran Branch, Iran
DOI : http://dx.doi.org/10.13005/ojc/310158
Article Received on :
Article Accepted on :
Article Published : 20 Mar 2015
An easily prepared Fe (ΙΙΙ)-derived Lewis acid ionic liquid, [C4 (mim) 2] (FeCl4)2, being comprised of dicationic ionic liquid cation and tetrachloroferrate anion, was found to be an efficient, recyclable catalyst for the synthesis of 1, 8-dioxooctahydroxanthenes by one-pot condensation reactions of dimedone/ 1, 3- cyclohexanedione with aromatic aldehydes under mild reaction conditions without utilization of additional organic solvent
KEYWORDS:Magnetic room temperature dicationic ionic liquid; 1; 8-dioxooctahydroxanthen; dimedone; Multi-component reactions; one-pot reaction
Download this article as:| Copy the following to cite this article: Mombaini-Goodajdar B, Kiasat A. R, Ezabadi A. One-pot green and efficient synthesis of xanthenedione derivativesusing [C4 (mim) 2](FeCl4)2as a magnetic room temperature dicationic ionic liquid. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Mombaini-Goodajdar B, Kiasat A. R, Ezabadi A. One-pot green and efficient synthesis of xanthenedione derivativesusing [C4 (mim) 2](FeCl4)2as a magnetic room temperature dicationic ionic liquid. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7860 |
Introduction
Xanthene derivatives are very important heterocyclic compounds and have been widely used as dyes fluorescent materials for visualization of bio-molecules and laser technologies due to their useful spectroscopic properties.1They have also been reported for their agricultural bactericide activity, photodynamic therapy, anti-inflammatory effect,and antiviral activity.2-5Due to their wide range of applications, these compounds have received a great deal of attention in connection with their synthesis.
Magnetic ILs not only have the excellent properties of IL but also exhibit an unexpectedly strong response to an additional magnet. These properties make magnetic ILs have more advantages and potential application prospects than conventional ILs in the fields of catalytic reactions, solvent effects and separation processes.11 [19]. Although several types of magnetic ionic liquids have been created in recent years, there have been only a few reports about their applications as catalyst in chemical transformations.
In continuation of our work on the catalytic properties of magnetic ionic liquids,12-13 herein, we wish to report a simple, convenient and efficient method for the use of magnetic room temperature dicationicionic liquid (MRTDIL)as catalyst for preparation of 1, 8- dioxo- octahydroxanthenes derivatives.
Experimental
Material and Methods
Melting points were measured on an Electrothermal 9100 apparatus and are uncorrected.1H &13 CNMR spectra were recorded on a Bruker Advanced DPX 400 MHz instrument spectrometer using TMS as the internal standard in CDCl3. IR spectra were recorded on a BOMEM MB-Series 1988 FT-IR spectrometer. Raman spectroscopy were recorded on a Bruker RFS 100/s Raman spectrometer. Aldehydes, and dimedone were purchased from Merck Company in high purity. Products were characterized by comparison of their physical and spectroscopic data with those of known samples.
Procedure for the preparation of [C4 (mim)2]Cl2(A)
1, 4-Dicholorobutane (1 mmol)was reacted with 1-methylimidazole (2 mmol), respectively, stirred in MeOH, refluxed for 24 h, and then precipitated from ethyl acetate to obtain the required product (white solid 1a, yield 94%).
Procedure for preparation of [Pbmim](FeCl4)2 as a magnetic room temperature dicationic ionic liquid
[C4(mim)2](FeCl4)2, MRTDIL, was prepared by mixing crystal powder of [pbmim]Cl2(1 mmol) with anhydrous FeCl3 (2 mmol) at room temperature for 3h, a dark brown liquid was obtained. The obtained MRTDIL was extracted with small amount of ethyl acetate. The solvent was evaporated and the resulting clear brown liquid was dried in vacuum oven at 60 °C for 24 h. The MRTDIL was obtained in high yield (89%).
General procedure for preparation of 1, 8-dioxo-octahydro-xanthenes (3a-l)
A mixture of dimedone/1, 3-cyclohexanedione (2 mmol), aromatic aldehyde (1 mmol) and MRTDIL (20% mol) was heated at 80 °C for an appropriate time (Table 2). After completion of the reaction (TLC), the mixture was cooled to room temperature and washed with cooled water. The solid product was purified by crystallization from aqueous EtOH to afford products 3a-3m (Scheme 2).
All the products were fully characterized by spectroscopic data and their melting points are compared with reported values.
Spectral data
9-(phenyl)- 3,3,6,6-tetramethyl – 3,4,5,6,7,9- hexahydro-1H-xanthene-1,8-(2H)-dione (3a): 1H
NMR (CDCl3, 400 MHz) δ: 1.02 (s, 6H), 1.13 (s, 6H), 2.19 (d, (2H, J = 16.2 Hz), 2.26 (d, 2H, J =16.2 Hz), 2.50 (s, 4H), 4.78 (s, 1H), 7.12 (t, 1H, J = 7.2 Hz), 7.24 (t, 2H, J = 7.5 Hz), 7.32 (d, 2H, J= 7.6Hz). 13C NMR(CDCl3, 100 MHz) δ: 27.75, 29.69, 32.26, 32.61, 41.29, 51.18, 116.07, 126.76, 128.45, 128.80, 144.54, 162.70,
Results and Discussion
The supported acidic PEG-MDIL catalyst was prepared according previously reported (Scheme 1).13
![Scheme 1. Synthesis of [C4 (mim) 2] (FeCl4)2 as a magnetic room temperature dicationic ionic liuquid](http://www.orientjchem.org/wp-content/uploads/2015/03/Vol31_No1_One_Bijan_sche1-150x150.jpg) |
Scheme1: Synthesis of [C4 (mim) 2] (FeCl4)2 as a magnetic room temperature dicationic ionic liuquid Click here to View Scheme |
2](http://www.orientjchem.org/wp-content/uploads/2015/03/Vol31_No1_One_Bijan_Fig1-150x150.jpg) |
Figure1: Visible spectrum of [C4(mim)2](FeCl4)2
|
Due to the paramagnetic nature of the [C4(mim)2](FeCl4)2, nuclear magnetic resonance technique could not be used to confirm its structure. Instead, UV spectra was used to characterize the [C4(mim)2](FeCl4)2 structure. The UV spectrum is shown in Figure 1. [C4(mim)2](FeCl4)2 spectra exhibited absorption bands in the visible region at 534, 620 and 680 nm which are characteristic for the FeCl4– anion.In order to be able to carry out preparation of 1, 8- dioxo- octahydroxanthenes derivatives in a more efficient way minimizing the time, temperature and amount of catalyst, the reaction of benzaldehyde, and dimedone was selected as model system to the effects of the catalyst at different reaction temperatures (25, 60, 80 and 100 °Cand the different amount of catalyst (0, 10, 20, 30, and 40% mol) were investigated. The reaction using 20% mol of MRTDIL at 80 °C proceeded in highest yield.
Further increase in temperature to, 100 °Chad little effect on the rate of reaction. Therefore, we kept the reaction temperature at 80 °C as optimal temperature.
This reaction was carried out without catalyst under solvent free conditions in order to establish the effectiveness of the catalyst. It was found that 1, 8- dioxo- octahydroxanthenes was not made after 1h of heating. The best results were obtained with 1: 2 ratio of benzaldehyde, dimedone, and 20% mol of MRTDIL after 10 min at 80 °C.
After optimizing the conditions, the generality of this process was demonstrated by the wide range of substituted aryl aldehydes to synthesize the corresponding products in excellent yields (Scheme 2, Table 1). As seen from Table 1, aromatic aldehydes having electron-donating as well as electron-withdrawing groups were uniformly transformed into the corresponding products in high yields within 10-15 min. Substituent on the aromatic ring had no obvious effect on yield or reaction time under the above optimal conditions. Unlike some previously reported methods; the present method does not require toxic organic solvents to produce the 1, 8-dioxo-octahydroxanthene derivatives.
The success of the above reactions prompted us to investigate the recyclability of catalyst. We carried out our study by using the reaction benzaldehyde with dimedone and under optimal conditions as a model study. For this aim, after completion of reaction (monitored by TLC), water was added to the reaction mixture and then the solid was isolated by filtration. The aqueous filtrate was then subjected to distillation at 80°C under reduced pressure (10 mmHg) for 4 h to recover the IL almost completely. The IL, thus recovered could bereused five times without loss of activity for the typical reaction (Fig 2).
The efficiency of MRTDIL (time, yield, reaction conditions) was compared with the efficiencies of other catalysts used in synthesis of 1, 8-dioxooctahydroxanthenes, the results are presented in Table 2. It is clear that the presented method is simpler, more efficient and less time consuming compared with other Methods.
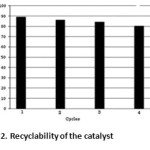 |
Figure2: Recyclability of the catalyst Click here to View figure |
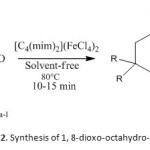 |
Scheme2: Synthesis of 1, 8-dioxo-octahydro-xanthenes Click here to View Scheme |
Table 1. Synthesis of xanthenes derivatives aIsolated yiel
| Entry | Ar | Product | R | Time (min) | Yield (%)a | M.P (°C)Found | Reportedref |
| 1 | C6H5 | 3a | CH3 | 10 | 92 | 203-204 | 204-20514 |
| 2 | 4-Cl-C6H4 | 3b | CH3 | 10 | 90 | 230-232 | 230-23114 |
| 3 | 4-Br-C6H4 | 3c | CH3 | 10 | 90 | 240-241 | 240-24215 |
| 4 | 2-Cl-C6H4 | 3d | CH3 | 10 | 91 | 225-227 | 224-22614 |
| 5 | 3-Cl-C6H4 | 3e | CH3 | 10 | 91 | 184-186 | 183-18414 |
| 6 | 4-NO2-C6H4 | 3f | CH3 | 10 | 89 | 222-223 | 22214 |
| 7 | 2-NO2-C6H4 | 3g | CH3 | 15 | 87 | 248-249 | 246-24814 |
| 8 | 3-NO2-C6H4 | 3h | CH3 | 15 | 89 | 171-173 | 170-17214 |
| 9 | 4-OCH3-C6H4 | 3i | CH3 | 15 | 88 | 241-243 | 242-24414 |
| 10 | 4-CH3-C6H4 | 3j | CH3 | 15 | 89 | 215-217 | 217-21814 |
| 11 | 4-CN-C6H4 | 3k | CH3 | 15 | 90 | 218-220 | 23016 |
| 12 | 4-CN-C6H4 | 3l | H | 15 | 89 | 268-270 | 273-27517 |
Table 2. Comparison of results using MDIL catalyst with results obtained by other workers
| Catalyst | Conditions |
Time (h) |
Yield % Rf |
| Silica sulfuric acidInCl3 .4H2OFe3+-montmorilloniteNaHSO4 -SiO2Amberlyst-15[C4(mim)2](FeCl4)2 | Solvent- free/80°CIonic liquid/80°CEtOH(reflux)CH3CN(reflux)CH3CN(reflux)Solvent-free/80°C |
1-2.5 4-10 6 6 5 0.08- 0.25 |
88-97 [9]76-95 [6]84-96 [10]90-98 [7]90-96 [8]87-91 – |
Conclusion
In conclusion, we have successfully developed a simple and green catalytic procedure for the efficient synthesis of xanthenes using MRTDIL and under mild reaction conditions. MRTDIL can replace the ILs and other homogeneous catalysts with reasonable recovery and reusability and therefore suitable for industrial applications.
Acknowledgments
We gratefully thank Islamic Azad University, Science and Research Branch Omidiyeh for financial support.
References
- Menchen, M. S.; Benson, S. C.; Lam, J. Y. L. et al., Patent, US, 6583168, 2003.
- Bhattacharya, A. K.; Rana, K. C.Mendeleev Commun. 2007, 17, 247–248.
- Ion, R. M.;Frackowiak, D.;Plannar, A.;Wiktorowicz, K. Acta. Biochim. Pol.1998, 45, 833-845.
- Hideu, T. Jpn. Tokkyo Koho JP 56005480 (1981) Chem. Abstr. 95, 80922b.
- Lambert, R. W.; Martin, J. A.; Merrett, J. H.; Parkes, K. E. B.;. Thomas, G. J., PCT Int. Appl. WO 9706178, 1997; Chem. Abstr. 1997, 126, p 212377y.
- Poupelin, J. P.; Saint-Rut, G.; Fussard-Blanpin, O.; Narcisse, G.; Uchida-Ernouf, G.;Lacroix, R. Eur. J. Med. Chem.1978, 13,67-71.
- Das, B.; Thirupathi, P.; Reddy, K. R.; Ravikanth, B.; Nagarapu, L. Catal. Commun. 2007, 8, 535-538.
- Das, B.; Thirupathi, P.; Mahender, I.; Reddy, V. S.; Rao, Y. K.J. Mol. Catal. A: Chem.2006, 247,233-239.
- Tisseh, Z. N.; Azimi, S. C.;Mirzaei, P.; Bazgir, A. Dyes Pigm.2008, 76, 836-839.
- Sharifi, A.; Abaee, M. S.; Tavakkoli, A.; Mirzaei,M.;Zolfaghari,A. Synth. Commun.2008, 38,2958-2966.
- Wang, J.; Yao, H.; Nie, Y.; Zhang, X.; Li, J. J. Mol. Liq.2012, 169, 152-155.
- Godajdar, M. B.; Kiasat, A. R.; Hashemi, M. M. Heterocycles, 2013, 87, 559.
- Godajdar, M. B.; Kiasat, A. R.; Hashemi, M. M. J. Mol. Liq.2013, 183, 14.
- Kantevari, S. K.; Bantu, R.; Nagarapu, L. J. Mol. Catal. A: Chem.2007, 269, 53-57.
- Fang, D.; Gong, K.; Liu, Z. L. Catal. Lett.2009, 127, 291-295.
- Shirini, F.; Imanzadeh, G. H.; Abedini, M.;AkberiDokhte Ghaziani, M.; Ghasemabadi, P. G.; Safarpoor Langroodi, M. Iranin Journal of Catalysis,2012, 2(3), 115-119
- Niknam, K.; Damya, M. J. Chin. Chem. Soc.2009, 56, 659-665.

This work is licensed under a Creative Commons Attribution 4.0 International License.









