Green Synthesis of Gold Nanoparticles
Hamid Reza Ghorbani*
Department of Chemical Engineering, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran
DOI : http://dx.doi.org/10.13005/ojc/310134
Article Received on :
Article Accepted on :
Article Published : 20 Mar 2015
There is an increased interest in understanding the toxicity and rational design of gold nanoparticles for biomedical applications in recent years. In this study gold nanoparticles were synthesized using dextrose as a reducing agent. The gold nanoparticles displayed characteristic Surface Plasmon Resonance peak at around 550 nm having a mean particle size of 75±30 nm. In order to identify and analyze nanoparticles, UV–Vis spectroscopy, Scanning electron microscopy (SEM), and dynamic light scattering (DLS) were used.
KEYWORDS:Gold Nanoparticles; Green Synthesis; Dextrose
Download this article as:| Copy the following to cite this article: Ghorbani H. R. Green Synthesis of Gold Nanoparticles. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Ghorbani H. R. Green Synthesis of Gold Nanoparticles. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7871 |
Introduction
Nanotechnology has emerged as the interdisciplinary science conjugating the basic concept of engineering with the application of biology, chemistry, physics and medical science [1]. Research in the field of synthesis of nanoparticles has been growing dramatically over the last few decades due to their unusual and highly interesting properties with wide potential applications like antifungal activity, antibacterial effect, skin cancer therapy etc. [2, 3]. Nanomaterials have potential applications in electronics and photonics, catalysis, information storage, chemical sensing and imaging, environmental remediation, drug delivery and biological labelling [4, 5, 6]. In particular, gold nanoparticles have many important applications that include: optical devices, electronics, biotechnologies and catalysis [4].
To obtain gold nanoparticles, various wet chemical methods employing various polar and nonpolar solvents have been used. The most common method is the reduction of tetrachloroauric acid (HAuCl4) by excess sodium borohydride (NaBH4) or sodium citrate in the presence of stabilizing/ capping ligands such as citrate, thiolates, amines, phosphanes, carbonyls, dendrimers, and surfactants [7, 8]. Size control in the small size range by UV irradiation is a simple, clean, reproducible and fast technique with very few control parameters that Sau et al. reported it [9]. In another work, uniform gold nanoparticles with low size polydispersity was synthesized from the chloroauric acid precursor at high concentration (2.5 mM) by the citrate reduction method via combined temperature and pH controls [10].
In another study, to synthesize gold nanoparticles, the reduction reaction between tetrachloroauric acid and sodium citrate was used. It was found that initial gold salt concentration and trisodium citrate concentrations and mixing rate are very important variables in the colloid nanoparticle synthesis [11]. In 2014, Verma et al. reported the synthesis of gold nanoparticles by trisodium citrate. Their work was carried out to investigate the synthesis and characterization of gold nanoparticles and the effect of various parameters (temperature, gold salt concentration trisodium citrate concentrations, mixing rate and pH) on its size and size distribution [12].
This paper presents the synthesis of gold nanoparticles by simple and green route. In this work, we employed HAuCl4 as an initial reagent, dextrose as reducing agent, and polyvinyl pyrrolidone (pvp) as a stabilizer.
Materials and methods
Materials
Hydrochloroauric acid trihydrate as the precursor, dextrose as the reducing agent and polyvinyl pyrrolidone (PVP) as the stabilizing agent to synthesize gold nanoparticles were purchased from Sigma-Aldrich.
Preparation
1 ml of aqueous solution of dextrose (3 g/100 ml) and 1 ml polyvinylpyrrolidone (20 g/l) were added to 100 ml aqueous solution of HAuCl4 (0.01 M). The reaction between this solution and Au3+ ions were carried out for about 15 min. After 15 min, the color of the reaction solution changed to vivid ruby red. This color change indicates a possibility of gold nanoparticles production.
Characterization of gold nanoparticles
Uv-vis spectroscopy was used to prove the existence of nanoparticles. The morphology and size was determined by the scanning electron microscopy (SEM), and Dynamic light scattering (DLS) analysis.
Results
For analytical study of the prepared sample, the amount of absorption within wave length of 500–650 nm was observed by uv-vis spectroscopy. It is known that an absorption band at about 520-580 nm due to surface plasmon resonance in gold nanoparticles [13]. Fig. 1 shows the UV-Vis spectra of Nano-gold Colloidal Solution recorded between 500 and 650 nm. As illustrated the SPR band cantered 550 nm confirms the formation of gold nanoparticles in the solution [12, 13].
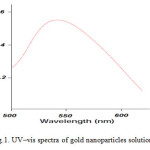 |
Figure1: UV–vis spectra of gold nanoparticles solution Click here to View figure |
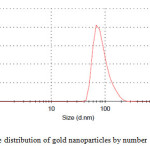 |
Figure2: The size distribution of gold nanoparticles by number Click here to View figure |
It is well known that gold can be reduced from Au3+ to Au0 by dextrose. Dynamic light scattering is a widely used technique for the determination of particle size in colloidal solution. As is seen in figure 2, average size of nanoparticle synthesized is 75 nm. The distribution of gold nanoparticles is about 30 nm which indicates moderate distribution of the nanoparticles. SEM analysis was carried out to understand the topology of gold nanoparticles. At the end, gold nanoparticles were studied by scanning electron microscopy (SEM). Fig. 3 confirms gold nanoparticles formation at nano size.
 |
Figure3: SEM micrograph of gold nanoparticles produced by dextrose Click here to View figure |
Conclusions
This is an environment friendly one-step method for synthesis of gold nanoparticles. The dextrose acted as a reducing agent of HAuCl4 and polyvinylpyrrolidone (pvp) as a capping material. Nanoparticles in the range of ~75 nm are synthesized by dextrose when it is added to HAuCl4. It seems this method is a fast and green method to form gold nanopartciles. The gold nanoparticles were characterized by UV-visible, TEM and DLS analysis.
References
- Prabhu, S.; Poulose, E.K. Int. Nano Lett., 2012, 2, 32.
- Alahmad, A.; Eleoui, M.; Falah, A.; Alghoraib, I. Phys Sci Res Int., 2013, 1(4), 89-96.
- Dong, P. V.; Ha, C. H.; Binh, L. T. Int. Nano Lett., 2012, 2, 9.
- R. Guo, Y. Song, G. Wang, R.W. Murray. J Am Chem Soc 2005, 127, 2752-2757.
- B. Huang, J. Zhang, J. Hou, C. Chen. Free Radic Biol Med. 2003, 35, 805–813.
- N. Durán1, P.D. Marcato, G. De Souza, O. Alves, E. Esposito. J Biomed. Nanotechnol. 2007, 3, 203–208
- Frens, G. Nat. Phys. Sci. 1973, 241, 20.
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. J. Am. Chem. Soc. 2007, 129, 13939–13948.
- Tapan K. Sau, Anjali Pal, N.R. Jana, Z.L. Wang and Tarasankar Pal, Journal of Nanoparticle Research, 2001, 3, 257–261.
- Chunfang Li, Dongxiang Li, Gangqiang Wan, Jie Xu and Wanguo Hou, Nanoscale Research Letters, 2011, 6, 440.
- Amir Tabrizi, Fatma Ayhan, Hakan Ayhan, Hacettepe J. Biol. & Chem., 2009, 37 (3) 217-226.
- Harihar Nath Verma, Praveen Singh and R. M. Chavan, Veterinary World, 2014, 7(2), 72-77.
- N. Sony, S. Prakash. Reports in Parasitology, 2012, 2, 1-7.

This work is licensed under a Creative Commons Attribution 4.0 International License.









