Comparative Physicochemical Evaluation of Fruits and Anti depressant Potential of volatile oils of fruits of Local Piper Species
Mohib Khan*
Oriental College of Pharmacy, Navi Mumbai
DOI : http://dx.doi.org/10.13005/ojc/310167
Article Received on :
Article Accepted on :
Article Published : 12 Mar 2015
In this study an attempt is made to evaluate physicochemical properties comparatively for the fruits of different Piper species available in the Mumbai region. The fruits of five species, viz. Piper betle Linn, Piper cubeba Linn. f., Piper retrofractum Vahl, Piper longum Linn and Piper nigrum Linn were evaluated comparatively for physicochemical properties, viz. Ash Value, Extractive Value, Loss on Drying, Mucilage Content, Crude Fibre Content, Volatile Oil Content and Piperine Content by Spectroscopic method. At the same time an attempt is made to evaluate antidepressant potential comparatively for the volatile oils of mentioned species, using forced swimming method, on albino mice with fluoxetine as standard antidepressant drug.
KEYWORDS:P. betle Linn; P. cubeba Linn. f.; Piper retrofractum Vahl; P. longum Linn. P. nigrum Linn. Clevenger Apparatus; UV-Spectrophotometer
Download this article as:| Copy the following to cite this article: Khan M. Comparative Physicochemical Evaluation of Fruits and Anti depressant Potential of volatile oils of fruits of Local Piper Species. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Khan M. Comparative Physicochemical Evaluation of Fruits and Anti depressant Potential of volatile oils of fruits of Local Piper Species. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7650 |
Introduction
Piper Linn., belonging to family Piperaceae is a very large genus of shrub,rarely herbs and trees, distributed throughout the tropical and sub-tropical regions of the world. About 30 species of the genus in India and 700 species in the world have been reported, of which, P. nigrum, the Black Pepper and P. betle Linn., the Pan or Betel are widely cultivated2. Five species are used as herbal ingredients of Asian medicines and they are P. betle , P. cubeba Linn. f. (Cubebs), P. retrofractum Vahl syn. P. chaba Hunter non Blume (Java Long Pepper), P. longum Linn. (Indian Long Pepper) and P. nigrum Linn3. The leaf juice of P. betle is used as eye drop4. P. cubeba is used as antibacterial5, expectorant6 and as gastroprotective7. P. longum is used as bioavailability enhancer8, digestive and in the treatment of bronchitis9 and also as hepatoprotective agent10. Scientists have received US patent on obtaining a diabetes mellitus therapeutic agent from P. longum11. P. nigrum is used as nervine tonic, and in the treatment of constipation, itching and flatulence12. Some of the Piper species contain a piperidine type alkaloid, piperine, which is a central nervous system depressant13. Most of the piper fruits contain volatile oils14.
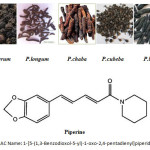 |
Figure 1 Click here to View figure |
Materials and methods
Collection of chemicals and plant material:
For the present study fruits of all the five selected species of Piper (P. betle Linn, P. cubeba Linn. f., Piper chaba (retrofractum) Vahl, P. longum Linn & P. nigrum Linn ), were collected from APMC Market, Vashi, Navi Mumbai, India. The fruits were identified, confirmed and authenticated by Prof. H.M. Pandit, Botany Dept., Khalsa College, Mumbai. Fruits of five Piper species were shade dried & ground to coarse powder. All reagents used in quantitative analysis and chemical investigation were of analytical grade and manufactured by E-Merck, Ranbaxy, Loba chemicals, S.D. fine chemicals and Yucca Enterprizes, Mumbai.
Physicochemical parameter
Determination of ash value14
Total Ash/ Water Soluble Ash/ Acid Insoluble Ash Value
For its detection, 2g of powdered material of each formulation and the individual ingredients of the powers were placed separately in a suitable tarred crucible of silica previously ignited and weighed. The powdered drugs were spread into an even layer and weighed accurately. The materials were incinerated by gradually increasing the heat, not exceeding 450°C until free from carbon, cooled in a desiccator, weighed and percentage ash was calculated by taking in account the difference of empty weight of crucible & that of crucible with total ash. The water soluble and acid insoluble ash value were then determined as per standard procedure.
Master Table
Determination of Solvent Extractive Value14
Water/Alcohol/Ether Soluble Extractive Value
About 5g of coarsely powdered air-dried drug was macerated with 100 ml of chloroform water/ alcohol/ ether respectively in three different closed flask for twenty-four hours, shaking frequently during six hours and allowed to stand for eighteen hours. These were then filtered rapidly; taking precautions against loss of solvent. 25 ml of the filtrate was evaporated to dryness in a tarred flat-bottomed shallow dish at 105°C to constant weight and weighed. The percentage of water / alcohol / ether soluble extractive were calculated with reference to the air dried drug and is represented as% value.
Master Table 1
Determination of Moisture Content14
About 2g of drug samples of each fruit was accurately weighed in a dried and tarred flat weighing bottle and dried at 105 0C for 5 hrs. Percentage was calculated with reference to initial weight.
Master Table 1
Determination of Mucilage Content15
About 1 gm of drug samples of each fruit/powder was accurately weighed and taken in a test tube. 10 ml of distilled water was added to it kept for 24 hours for maceration. On the next day fruit/powder solutions were filtered. The filtrate then treated with 5 ml of ethanol. The mucilage appeared in the ethanol which was then filtered through an already weighed watmann filter paper and difference in the weight was then calculated.
Master Table 1
Determination of Crude Fibre Content16
About 2 gm of drug sample extracted with 50 ml of methylene chloride. Filtered and to the residue added 50 ml of dilute sulphuric acid, boiled for 30 minutes, filtered. The residue then washed with water. Ignited already weighed crucible and contents/residue in an electric muffle furnace at 600°C. Then difference in weight was calculated.
Master Table 1
Determination of Volatile Oil Content17
About 100 gms of drug samples of each fruit was accurately weighed and transferred to a 500ml round bottom flask. Sufficient amount water was added and a Clevenger Apparatus then attached to the RBF with condenser and mixture was then heated for six hour for so as to isolate the volatile oil. The volatile oil of individual drug was then collected in graduated tube of Clevenger Apparatus and separated and stored in vials.
Master Table 1
Determination of Piperine Content18
Instrument
UV Spectro-photometer: UV-1800-Shimatzu
Preparation of standard solution of Piperine
Accurately weighed Piperine (10 mg) was transferred in 100 ml volumetric flask and dissolved in & diluted to 100 ml with ethanol. The final solution contained 100 mg of the Piperine per ml of the solution.
Preparation of Piperine extract of different Piper Extracts
Accurately weighed 1 gm of Piper fruit powder reflux with 40 ml of ethanol for 1 hour. Filtered the extract and re-reflux the marc I left with 30 ml of ethanol for another 1 hours. Filtered and combined the previous filtrate. Further reflux the mark II left with 20 ml of ethanol. Again filtered and combined filtrate with previous filtrates, finally make up the volume up to 100 ml with ethanol in a volumetric flask.
Preparation of calibration curve for Piperine
Standard solutions of Piperine (0.1, 0.2, 0.3, 0.4 and 0.5 ml) were pipetted in a series of five 10 ml volumetric flask so as to give concentration range 1-5 ug/ml The absorbance of the Piperine was measured at 342.6 nm against ethanol. The concentration and then the percentage of Piperine in different Piper fruits were calculated.
Master Table 1
Master Table 1: Physicochemical Evaluation of Fruits of Local Piper Species
|
Sr. No. |
Name of the Drug |
Ash Value |
Extractive Value |
Moisture Content |
Fruit Mucilage Content
|
Crude Fibre Content |
Volatile Oil Content |
Piperine Content (gm percent)
|
||||
|
T.A.V. |
W.S.A.V. |
A.I.A.V. |
W.S.E.V. |
A.S.E.V. |
E.S.E.V. |
|||||||
|
1 |
Piper nigrum |
10.8±0.4 |
8.8±0.2 |
1.0±0.1 |
2.5±0.1 |
6.2±0.2 |
5.8±0.2 |
1.80 |
3.8 |
0.35 |
1.6 |
1.70 gm % |
|
2 |
Piper longum |
5.7±0.2 |
3.2±0.1 |
1.5±0.1 |
18.5±0.4 |
6.8±0.2 |
9.8±0.2 |
1.35 |
7.9 |
0.45 |
0.2 |
0.30 gm % |
|
3 |
Piper chaba |
6.85±0.8 |
3.6±0.1 |
3.0±0.1 |
6.0±0.2 |
8.6±0.2 |
7.2±0.2 |
1.25 |
8.6 |
0.15 |
0.8 |
0.95 gm % |
|
4 |
Piper cubeba |
5.9±0.55 |
2.8±0.1 |
3.0±0.1 |
7.5±0.2 |
26.0±0.4 |
23.8±0.3 |
1.75 |
4.4 |
0.45 |
4.4 |
0.15 gm % |
|
5 |
Piper betle |
7.65±0.5 |
4.6±0.2 |
2.0±0.1 |
11.5±0..3 |
16.0±0.3 |
5.6±0.2 |
1.65 |
9.1 |
0.35 |
0.2 |
0.90 gm % |
T.A.V.-Total Ash Value, W.S.A.V.-Water Soluble Ash Value, A.I.A.V.-Acid Insoluble Ash Value
W.S.E.V.Water Soluble Extractive Value, A.S.E.V. Alcohol Soluble Extractive Value & E.S.E.V.Ether Soluble Extractive Value
Antidepressant Activity19-22
Forced Swimming Method
The forced swimming test is adopted here is a modification of the method described by Porsolt et al. (1977). Mice were individually forced to swim for 15 min in glass cylinders (height: 20 cm, diameter: 14 cm), containing 10 cm of water at 25 °C, which is a pre-test, and then mice were removed and dried before being returned to cages. Then standard Fluoxetine and essential oil under tests were administered. Four hours later, mice were placed in the cylinders again for a 6-min test in the same system depicted above. The duration of immobility was recorded during the last 4 min of the 6-min testing period. Groups of 6 mice were treated with vehicle (10 mg/kg, p.o.), drug-treated groups (10 ml/kg, p.o.), Fluoxetine (1 mg/kg,i.p.). Table 2
Table 2: Antidepressant Activity of Essential Oils of Piper Fruits: Forced Swimming Method
|
Normal control |
Standard |
T-1 |
T-2 |
T-3 |
T-4 |
T-5 |
|
|
Body weight (g) |
49.33 ± 2.06# | 60.67 ± 3.53* | 69.50 ± 1.78** | 64.17 ± 2.17** | 55.33 ± 2.40 | 53.17 ± 4.31 | 56.67 ± 2.95 |
|
Mobility (second) |
239.00 ± 17.03## | 293.50 ± 11.83**, | 338.83 ± 6.75**, ## | 334.17 ± 4.02**, # | 332.17 ± 4.92**, # | 348.83 ± 3.02**, ## | 350.5 ± 0.76**, ## |
|
Immobility (second) |
121.67 ± 17.36## | 66.50 ± 11.83** | 21.16 ± 6.745**, ## | 25.83 ± 4.02**, # | 27.83 ± 4.92**, # | 11.16 ± 3.02**, ## | 9.50 ± 0.76**, ## |
Values are represented as Mean ± SEM, one way ANOVA followed by Dunnett’s test for multiple comparisons
*P<0.05, **P<0.01 vs. normal control.
#P<0.05, ##P<0.01 vs. standard.
Result and Discussion
In the exhaustive study of Piper fruits, the conclusions drawn are; (a) Piperine content in Piper nigrum is maximum followed by Piper chaba and minimum in Piper cubeba. Here an easy UV Spectroscopic method is used for Piperine determination (b) Volatile oil content is in maximum amount in Piper cubeba followed by Piper nigrum and Piper chaba and least in remaining two species of Piper. Here hydrodistillation method is used for isolation of Volatile oils. (c) Crude fibres are fat free organic substances which are insoluble in acid and alkaline media. The crude fibre content estimation showed that Piper longum and Piper cubeba have equal and maximum crude fibre content followed by Piper nigrum and Piper betle in equal amount. (d) The mucilage content in Piper betle was in maximum amount followed by Piper chaba and then Piper longum. It is revealed that circular or rounded shaped fruit do not contain mucilage in large amount. (e) It is found that moisture content in Piper nigrum is in maximum amount while in Piper chaba it in least amount.
All volatile oils of Piper fruits have shown more activity than the standard compound. Fluoxetine, which is a Selective Serotonin Reuptake inhibitor was taken as standard, therefore for all these oils which have shown comparable and more activity to that of Fluoxetine should be further studied with different models of Antidepressant Activity.
Acknowledgement
The author is thankful to Mr.Waseem Khan, Managing Director, Oriental Education Society for a suitable financial assistance and permission to carry out the work on the Piper species available in local market. The author is also thankful to Mr.Amjad Ali, Mr. Imtiyaz Ansari, Ms.Poonam Sarang, Ms.Sonal Girkar and Nilesh Kharwate, Mr.Altamash, Mr. Amogh and Ms. Jyoti, for their help in the Laboratory.
References
- Khan, Mohib* and Siddiqui, M., Nat. Pdt. Rad. Vol. 6(2), pp.111-113 (2007).
- Kirtikar, K.R. and Basu, B.D., Indian Medicinal Plants, International Book Distributors, Reprint, Vol. 3, pp. 2126-2136 (1999).
- Evans, W.C. In : Pharmacognosy, 15th Edn, WB Saunders Company Ltd., London, pp. 545–546 (2002).
- Kumar, S. The Medicinal Plants of North-East India, Scientific Publishers, 150 (2002).
- The Indian Herbal Pharmacopoeia, Indian Drugs Manufacturers’ Association, Mumbai, Revised Edn, pp. 304, (2002).
- The Wealth of India: A Dictionary of the Indian Raw Materials and Industrial Products — Raw Material Series, Publications and Information Directorate, CSIR, New Delhi, Vol. 8, pp.93 (1969).
- Morikawa, T., Matsuda, H., Yamaguchi, I., Pongipiriyadacha, Y. and Yoshikawa, M. Planta Med, 70 (2), 152-159 (2004).
- Quality Standards of Indian Medicinal Plants, ICMR, New Delhi, Vol.1, 172 (2003)
- Sathiyamoorthy, P. and Elumalai, E. Herbal Tech Ind, 2 (9), 13 (2006)
- Jalalpure, S.S., Patil, M.B. Prakash, N.S., Hemlata, K. and Manvi, F.V. Indian J Pharm Sci , 65(4), 363-366 (2003).
- Mathew, J.C. Chronicle Pharmabiz , 6 (36), 2 (2006).
- Kurian, J.C. Plants That Heal, 3rd Edn, Oriental Watchman Publishing House, Pune, pp. 251-252 (1999)
- Bruneton, J. Pharmacognosy and Phytochemistry Medicinal Plants, 2nd Edn, Lavoisier Publishing Inc., USA, pp. 862 (1993).
- Kokate, C.K., Purohit, A.P. and Gokhale, S.B., Pharmacognosy, 27th Edn, Nirali Prakashan, pp. 352-353 (2004).
- Woods, D.L. and Downey, R.K., Can. J.Pharm.Sci., pp. 1031-1033 (1980).
- Anonymous, India-Analysis of crude fibre, Report of the 14th meeting of the committee on Quality, Rex Hotel, H.C.M.City, Vietnam, pp.77-79 (2008)
- Khandelwal, K.R., Practical Pharmacognosy, Nirali Prakashan, 20th Edition, August, pp 23.16 (2010).
- Jain, T. and Dashora, K. Asian J. Pharm. Tech. Vol. 2(1), pp 01-03 (2012).
- L M Lopes C, Gonçalves e Sá C, de Almeida AA, da Costa JP, Marques TH, Feitosa CM, Saldanha GB, de Freitas RM, Sedative, anxiolytic and antidepressant activities of Citrus limon (Burn) essential oil in mice, Pharmazie. 2011 Aug;66(8):623-7.
- Sah, S. P., Mathela, C.S. and Chopra, K. Involvement of nitric oxide (NO) signalling pathway in the antidepressant activity of essential oil of Valeriana Wallichii patchouli alcohol … Journal of Phytotherapy & Phytopharmacology.
- Emamghoreishi M., Talebianpour M.S. Antidepressant effect of Melissa officinalis in the forced swimming test, DARU Vol. 17, No. 1 2009.
- Chunyan, Y., Antinociceptive, antidepressant, anxiolytic and toxicity studies on Piper laetispicum C. DC. Ph. D. Dissertation, 2009.

This work is licensed under a Creative Commons Attribution 4.0 International License.









