Antibacterial effects of silver nanoparticles on Escherichia coli and Bacillus subtilis
Hamid Reza Ghorbani1* and Saeid Soltani2
1Department of Chemical Engineering, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran
2Department of Biology, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran.
DOI : http://dx.doi.org/10.13005/ojc/310139
Article Received on :
Article Accepted on :
Article Published : 23 Feb 2015
Metal nanoparticles have been intensively studied within the past decade. Nanosized materials have been an important topic in basic and applied sciences. In this work we synthesized silver nanoparticles by Salmonella typhirium. The silver nanoparticles displayed characteristic Surface Plasmon Resonance peak at around 422 nm. In addition, the morphology of nanoparticles was observed by transmission electron microscopy. The size of the nanoparticles was determined to be 65 nm, applying dynamic light scattering. The main objective of this work is the study of antibacterial effects of silver nanoparticles. Silver nanoparticles showed high antibacterial activity against Escherichia coli and Bacillus subtilis bacteria.
KEYWORDS:Silver; Nanoparticles; Antibacterial; E; coli; B; subtilis
Download this article as:| Copy the following to cite this article: Ghorbani H. R, Soltani S. Antibacterial effects of silver nanoparticles on Escherichia coli and Bacillus subtilis. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Ghorbani H. R, Soltani S. Antibacterial effects of silver nanoparticles on Escherichia coli and Bacillus subtilis. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7344 |
Introduction
The synthesis of nanoparticles of different chemical compositions, sizes, and controlled monodispersity is an important area of research in nanotechnology. Among noble metal nanoparticles, silver nanoparticles have a wide area of interest, thus, they have gained immense applicability into the daily life of modern individuals such as for biomedical applications, air and water purification, food production, cosmetics, garments and various household products [1]. There are several methods for fabrication of silver nanoparticles. Generally, silver nanoparticles can be prepared by chemical methods such as chemical reduction and electrochemical techniques, physical methods such as Arc-discharge and physical vapor condensation and biological methods such as the use of microorganisms [2, 3, 4, 5]. The biological route of synthesis provides a great diversity in choice for its raw materials such as bacteria, algae, fungi and plants [6].
In 2011, Veerasamy et al. reported a simple and eco-friendly method to synthesis of silver nanoparticles using Garcinia mangostana leaf extract as reducing agent. The aqueous silver ions when exposed to leaf extract were reduced and resulted in silver nanoparticles whose average size was 35 nm [7]. In another study, extra cellular synthesis of silver nanoparticles using Aspergillus flavus NJP08 was carried out. The average particle size was found 17 ± 5.9 nm using Dynamic Light Scattering [8]. Shameli et al. present green biosynthesis of silver nanoparticles using Curcuma longa tuber powder [9]. In 2014, synthesis of silver nanoparticles from silver(I) reduction by the periplasmic nitrate reductase c-type cytochrome subunit NapC in a silver-resistant E. coli was reported [10].
This paper presents the biosynthesis of silver nanoparticles and the study of antibacterial effects. In this work, we employed silver nitrate as an initial reagent and Salmonella typhirium as reducing agent. The antibacterial activity of silver nanoparticles was tested against Escherichia coli and Bacillus subtilis by disc diffusion method.
Materials and Methods
Preparation
We used previously described method to synthesize silver nanoparticles using Salmonella typhirium. The culture was centrifuged at 5000 rpm for 15 minutes and the supernatant was used for the synthesis of silver nanoparticles. 1 ml of supernatant was added separately to the reaction vessel containing 100 ml AgNO3 at a concentration of 0.001 M. The reaction between this supernatant and Ag+ ions were carried out in bright conditions for 5 minutes.
Characterization of Silver Nanoparticles
Uv-vis spectroscopy was used to prove the existence of nanoparticles. The morphology and size was determined by the transmission electron microscopy (TEM), and Dynamic light scattering (DLS) analysis.
Disc Diffusion Method for Antimicrobial Activity
The antibacterial activities of Ag nanoparticles were carried out by disc diffusion method.Nutrient agar medium plates were prepared, sterilized and solidified. After solidification bacterial cultures were swabbed on these plates. Samples of Ag nanoparticles, two antibiotics (colistin and tetracycline) and control sample placed (supernatant) in the nutrient agar plate and kept for incubation at 37°C for 24 hours. Zones of inhibition for control sample, Ag nanoparticles and two antibiotics were measured. The experiments were repeated four times and mean values of zone diameter were presented.
Results
For analytical study of the prepared sample, the amount of absorption within wave length of 350–600 nm was observed by UV-Vis spectroscopy. The Ag nanoparticles displayed characteristic Surface Plasmon Resonance peak at around 422 nm (Fig. 1). In order to study the size of the produced silver nanoparticles, dynamic light scattering analysis was used. As is seen in figure 2, average size of nanoparticle synthesized is ~65 nm. In addition, the Ag nanoparticles synthesized were studied by transmission electron microscopy (TEM) (Fig.3).
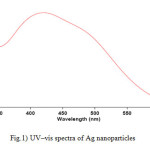 |
Figure1: UV–vis spectra of Ag nanoparticles Click here to View figure |
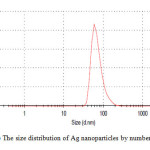 |
Figure2: The size distribution of Ag nanoparticles by number Click here to View figure |
 |
Figure3: TEM micrograph of Ag nanoparticles Click here to View figure |
The silver nanoparticles show an effective antibacterial activity against E. coli and B. subtilis. The result suggests that silver nanoparticles undergo an interaction with bacterial cell and displayed the strong action against E.coli, and B. subtilis. The formation of clear zone (restricted bacterial growth) around the cavity is an indication of antibacterial activity (Fig. 4). As can be seen, the antibacterial activity of silver nanoparticles on B. subtilis and E. coli is more than tetracycline and colistin on B. subtilis and E. coli. The zone of inhibition of diameters was determined. The results have been summarized in Tables 1.
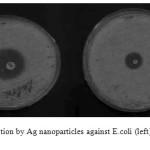 |
Figure4: Zone of inhibition by Ag nanoparticles against E.coli (left) and B. subtilis (right)
|
Table 1) Antibacterial activity of Ag nanoparticles, zone of inhibition (mm)
|
Control sample |
Silver nanoparticles |
Colistin |
Tetracycline |
Organism
|
|
– |
19 |
6 |
10 |
E.coli |
|
– |
9 |
5 |
10 |
B. subtilis |
Conclusions
Silver nanoparticles were successfully synthesized by Salmonella typhirium. The characterization with UV-visible spectroscopy, Dynamic light scattering (DLS), and transmission electron microsopy (TEM) confirms the synthesis of pure, stable and crystalline silver nanoparticles. The antimicrobial assay showed that silver nanoparticles presented good antibacterial performance against highly contagious bacterial. Microorganisms were Bacillus subtilis and Escherichia coli. The antibacterial activity performance of silver nanoparticles was done by using disc diffusion method.
References
- Marambio-Jones, C.; Hoek, E. M. J. Nanopart Res., 2010, 12(5), 1531–1551.
- Lanje1, A. S.; Sharma, S. J.; Pode, R. D. J. Chem. Pharm. Res., 2010, 2(3), 478-483.
- Mani, U.; Dhanasingh, S.; Arunachalam, R.; Paul, E.; Shanmugam, O.; Rose, C.; Mandal, A. B. Prog Nanotechnol Nanomater, 2013, 2 (1), 21-25.
- Moazeni, M.; Shahverdi, A. R.; Nabili, M.; Noorbakhsh, F.; Rezaie, S. Nanomedicine Journal, 2014, 1(4), 267-275.
- Mohan, S.; Oluwafemi, O. S.; George, S. C.; Jayachandran, V. P.; Lewu, F. B.; Songca S. P.; Kalarikkal, N.; Thomas, S. Carbohydr Polym, 2014, 15, 469-474.
- Mittal, A. K.; Chisti, Y.; Banerjee, U. C. Biotech. Adv. 2013, 31(2), 346-356.
- Veerasamya, R.; Xin, T.Z.; Gunasagaran, S.;Xiang,T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. J. Saudi Chem. Soc. 2011, 15(2), 113–120.
- Jain, N.; Bhargava, A.; Majumdar, S.; Tarafdar, J. C.; Panwar, J. Nanoscale, 2011, 3, 635-641.
- Shameli, K.; Ahmad, M.B.; Zamanian, A.; Sangpour, P.; Shabanzadeh, P.; Abdollahi, Y.; Zarga, M. Int J Nanomedicine, 2012, 7, 5603—5610.
- Lin, I.; Lok, C.; Che,C. Chem. Sci., 2014,5, 3144-3150.

This work is licensed under a Creative Commons Attribution 4.0 International License.









