Synthesis of Benzothiazinophenothiazine Derivatives and their Antimicrobial Screening
Emmanuel O. Adekola1, Benjamin E Ezema1, Jude I. Ayogu1, David I. Ugwu1*, Chidimma G. Ezema2, Abuekwu P. Nwasi1 and Christian O. Ike1
1Department of Pure and Industrial Chemistry,Faculty of Physical Sciences,University of Nigeria, Nsukka, Synthetic Organic Chemistry Unit, 41002, Nigeria
2National Centre for Energy Research and Development, University of Nigeria, Nsukka.
DOI : http://dx.doi.org/10.13005/ojc/300406
Article Received on :
Article Accepted on :
Article Published : 18 Dec 2014
The synthesis of benzothiazinophenothiazine derivatives from simple heterocyclic compounds was thoroughly investigated. The intermediate, 6-chloro-5H-benzo[a]phenothiazin-5-one was afforded by the condensation of 2-aminothiophenol with 2,3-dichloro-1, 4-naphthoquinone in an alkaline medium. Further condensation of the intermediate with 2,4-diamino-6-hydroxypyrimidine-5-thiol obtained by alkaline hydrolysis of 2,4-diamino-6-hydroxy-5-thiacyanatopyrimidine gave 7-amino-9-hydroxy-6-8,diazabenzo[a][1,4]benzothiazino[3,2-c]phenothiazine. However, facile acid-catalyzed synthesis of four other benzothiazinophenothiazine ring systems was accomplished with improved yield and lesser reaction time. The Structural confirmation was done using UV-Visible spectroscopy, FT-IR, 1H and 13C-NMR and elemental analysis. The synthesized compounds were screened against some micro-organisms and the results showed that the complex derivatives were significantly active against the microorganisms.
KEYWORDS:benzothiazinophenothiazine derivatives; antimicrobial screening; Sensitivity test; minimium inhibition concentration; inhibition zone diameter
Download this article as:| Copy the following to cite this article: Adekola E. O, Ezema B. E, Ayogu J. I, Ugwu D. I, Ezema C. G, Nwasi A. P, Ike C. O. Synthesis of Benzothiazinophenothiazine Derivatives and their Antimicrobial Screening. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Adekola E. O, Ezema B. E, Ayogu J. I, Ugwu D. I, Ezema C. G, Nwasi A. P, Ike C. O. Synthesis of Benzothiazinophenothiazine Derivatives and their Antimicrobial Screening. Available from: http://www.orientjchem.org/?p=6037 |
Introduction
Phenothiazines are amongst the most frequently encountered bioactive heterocycles in compound of biological interest.1 Phenothiazine derivatives have been shown to possess a broad spectrum of pharmacological activity depending on their particular structure like antiparkinsonian2-3, tranquilizer4, anti-inflamatory5-7, antimalarial8-9, antipsychotic, 10-12, antimicrobial 13-15, anti-tubercular 16-18, antitumor19-20, antihistaminic21-22, analgesic 23 , prion disease drug24 . In textile, paint and plastic industries, they are used as dyes and pigments25-26 and in agricultural industries as insecticides.27 In petroleum industries, they are found useful as antioxidants in lubricants and fuels.28 it has been observed that some phenothiazines inhibit intracellular replication of viruses including human Immunodeficiency viruses (HIV) 29. On the other hand, some have been reported to exhibit significant anticancer activity30-31. Owing to the wide range of applications of phenothiazine, intensive research has been in progress for more derivatives with highly improved pharmacological and biological activities. Hence, several papers describing the successful synthesis of these derivatives had been reported especially on the angular derivatives including the non-aza and the congeneric aza-analogues, but there are still limited literatures on the complex derivatives of this phenothiazine ring system.The past work done was based on their dye and pigment properties, not much is known of their antimicrobial properties. Therefore, the authors describe the synthesis of complex aza derivatives of benzothiazinophenothiazine and their antimicrobial screening.
Experimental Section
All chemicals used were of laboratory grade (Sigma Aldrich). The melting points were determined with a Fischer John’s apparatus and were uncorrected. UV/Visible spectra were recorded on UV-25500PC series spectrophotometer using matched 1 cm quartz cells. The IR spectra were recorded on 8400S FT-IR spectrometer using KBr discs (at NARICT, ZARIA). The 1H-NMR and 13C-NMR were scanned at university of stratchclyde, Scotland on a Jeol FX 90Q spectrometer using TMS as internal standard (chemical shift in δ).. Elemental analysis was carried on CHN rapid analyzer and the antimicrobial screening was done at the Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka.
Synthesis of 6-chloro-5H-benzo[a]phenothiazin-5-one (1)
To a mixture of 2-aminothiophenol (4.0 g, 32 mmol) and anhydrous sodium trioxocarbonate (IV) (3.3 g, 31 mmol) in a 250 mL two-necked flask equipped with magnetic stirrer, thermometer and reflux condenser, was added a solution of benzene (100 mL) and DMF (10 mL). The mixture was boiled for 1 h and thereafter, 2,3-dichloro-1,4-naphthoquinone (7.26 g, 32 mmol) was added and the entire solution refluxed with continuous stirring for more 7 h at 78-80 oC. Then, the solvent was distilled off and the slurry poured into water and stirred for 20 min to dissolve the inorganic materials. It was left overnight, filtered and recrystallized from methanol-acetone mixture to obtain a purple microcrystalline powder of 1 (8.5 g, 85% yield). m.p 234 oC; Uv-V (MeOH)λmax (nm) log( ): 210 (1.978), 221 (1.4779), 228 (1.2517), 234 (1.3543), 247 (0.2255), 255 (0.5149), 261 (1.4326), 266 (1.2138), 289 (2.3308), 314 (2.3640), 379 (2.3041), 491 (2.2271); FT-IR (KBr): νmax 3063 cm-1 (aromatic C-H str.), 1631 cm-1 (C=O), 1502 (aromatic C=N), 1467 (aromatic C=C) 780 (C-Cl), 1292, 1228, 1155, 1082, 1039, 902, 680, 567 and 462 cm-1.
Synthesis of 7-amino-9-hydroxy-6, 8-diazabenzo[a][1,4]benzothiazino[3,2-c]phenothizine (2)
The same procedure as in compound 1 above was employed using 2,4-diamino-6-hydroxypyrimidine-5-thiol (1.0 g, 6 mmol) and anhydrous sodium trioxocarbonate(IV) (1.5 g, 14 mmol) but 6-chloro-5H-benzo[a]phenothiazin-5-one (1.8 g, 6 mmol) in the second stage instead of 2,3-dichloro-1,4-naphthoquinone to give 2 (2.46 g 84 % yield) as purple-red crystals; m.p. > 300 oC; Uv-V (MeOH) λmax (nm) log ): 780 (1.7000), 745 (1.7204), 688 (1.7704), 486 (2.5758), 380 (2.6022), 319 (2.6437), 265 (2.8636), 254 (2.7968), 247 (2.7875); IR (KBr): νmax 3761 (OH), 3400, 3331 ( >N-H str) 3063, (aromatic C-H str.), 1608 (arom. C=N) 1494, 1452 (arom. C=C), 1300, 1242, 1151, 1087, 1024, 877, 850, 758, 673, 642, 538 and 455 cm-1; 1H-NMR (DMSO: d6) δ: 8.87 (2H, dd, J1 = 7.87, J2 = 1.36 Hz), 8.24 (2H, dd, J1= 7.55, J2=1.61 Hz). 8.08 (4H, m, Ar-H), 7.92-7.67 (4H, m, 1-H, 2-H, 3-H), 5.46 (1H, s, 9-OH); 13C-NMR (DMSO) δ: Few peaks, due to the insolubility of the compound. Absence of chemical shift at 150 ppm and above indicates the absence of C=O. Analysis calculated for C20H11N5OS2: C, 59.84, H, 2.76, N, 17.44 and S, 15.97. Found: C, 59.88, H, 2.81, N, 17.45 and S, 15.99.
Synthesis of 7,14-diethylthio-9,12-dihydroxy-6,8,13,15-tetraazabenzo[a][1,4]benzothiazino[3,2-c]phenothiazine. (3)
To 4-amino-2-ethylthio-6-hydroxypyrimidine-5-thiol (2 g; 10 mmol) in 250 mL two-necked flask equipped with magnetic stirrer, thermometer and a reflux condenser, was added absolute ethanol (100 mL) and 15 % HCl (10 mL ). The mixture was warmed to dissolve the solid, 2,3-dichloro-1,4-naphthoquionone (1.1 g; 5 mmol) was added and the mixture refluxed on a water bath for 6 h at 78 oC. On refluxing, an immediate appearance of orange brown ppt was observed which turned blue and persisted. Thereafter, the mixture was poured in cold water, stirred, filtered and the residue crystallized from acetone-methanol mixture to obtain 3 (3.2 g; 91 % yield) as blue powder. m.p > 310 oC; Uv-V (MeOH) λmax (nm) log( ): 748 (1.2533), 539 (2.4100), 328 (3.2316), 322 (3.2249), 305 (3.2634), 276 (1.5901), 266 (1.7812), 257 (1.0315), 239 (1.0895), 233 (0.9881), 288 (0.8554), 222 (1.3721); IR (KBr) νmax 3755, 3400 (OH), 3184 (aromatic C-H str.), 2937 (C-H str of CH3 or CH2), 1626, 1556, (aromatic C=N), 1518, 1446 (aromatic C=C), 1325, 1276, 1224, 1130, 1095, 964, 885, 823, 779, 700, 648, 561, 447 cm-1; 1H-NMR (DMSO: d6) δ: 8.08 (4H, m, Ar-H), 7.91 (4H, td, J1 = 6.72, J2 = 5.89, J3 = 3.44 Hz, Ar-H),3.3 (2H, q, J1 = 8.16, J2 = 7.3 Hz, -SCH2CH3) 3.06 (3H, t, -SCH2CH3), 1.33 (3H, dt, J2 = 54.74, J2 = 7.41 Hz); 13C-NMR (DMSO) δ: 142.98-127.63 (C1 – C9, Ar-C), 15.19 (aliphatic carbon). Analysis: Calculated for C19H11N7O2S2: C, 52.65, H, 2.56, N, 22.62, S, 14.79. Found: C, 52.72, H, 2.59, N, 22.60, S, 14.90.
Synthesis of 8,3-dimethoxy-9,12-diazabenzo[a][1,4]benzothiazino[3,2-c]phenothiazine (4)
3-Amino-6-methoxypyridin-2-thiol (2 g; 13 mmol) in absolute ethanol (100 mL) was placed in 2-necked reaction flask containing 10 mL of 15 % HCl and warmed. 2,3-dichloro-1,4-naphthoquinone (1.45 g, 6 mmol) was then added and the mixture refluxed on a water bath for 6.5 h at 78 oC. The initially formed yellow precipitate turned dark-brown which persisted throughout the reflux period. The mixture was then poured into a beaker and heated for 15 min to evaporate the remaining solvent. It was diluted twice with water and cooled. The dark-brown residue was collected after filtration, and crystallization from acetone-methanol mixture gave a brown microcrystalline powder of 4 (3.40 g; 94 % yield). m.p > 310 oC; Uv-V (MeOH) λmax (nm) log( : 749 (1.6377), 478 (2.5529), 334 (3.0278), 296 (2.8135), 275 (1.2669), 269 (1.5525), 257 (1.0313), 246 (1.1897), 239 (1.4659), 228 (1.3409), 222 (1.5827), 212 (1.2566), 203 (1.6419); IR (KBr) 3088 (aromatic C-H str.), 1587 (C=N aromatic), 1504 (aromatic C=C), 1346, 1278, 1147, 1087, 1022, 889, 831, 781, 692, 648, 555 and 462 cm-1;1H-NMR (DMSO: d6) δ: 10.57 (2H, d), 10.31 (2H, d, J = 7.51 Hz), 7.66 (4H, s, Het. Ar-H), 7.31 (4H, s, Ar-H), 4.28 (3H, d, -OCH3).13C-NMR (DMSO) δ: There were few peaks due to difficulty in the solubility of the compound; 24.63-15.05 ppm (aliphatic carbon). Analysis: Calculated for C22 H14N4O2S2: C, 61.38, H, 3.28, N, 13.01, S, 14.90. Found: C, 61.45, H, 3.33, N, 13.21, S, 15.10.
Synthesis of 6,13-dichloro-3, 10-diethylthio-1,8-dihydroxy-2,4,9,11-tetrazatriphenodithiazine (5)
A mixture of 4-amino-2-ethylthio-6-hydroxypyrimidine-5-thiol (4 g; 19 mmol), absolute ethanol (150 mL) and 10 mL of 15 % HCl was warmed and tetrachloro-1,4-benzoquinone (2 g; 8 mmol) was then added. The mixture was refluxed on a water bath with continuous stirring for 6 h. An immediate formation of a yellow clear solution and then observation of yellowish-red precipitate occurs. At the end of the reflux period, the mixture was poured into a beaker, filtered and recrystallized from aqueous acetone to obtain a greenish yellow powder, 5 ((5.3,73 % yield g). m.p > 310 oC; Uv-V (MeOH) λmax (nm) log( : 753 (1.1864), 671 (1.2357), 612 (1.5859), 531 (1.5920), 491 (1.6538), 322 (3.2634), 305 (3.2993), 274 (1.1819), 257 (1.4759), 251 (1.7954), 246 (1.2910); IR (KBr): 3751, 3375 (OH), 3196, (C-H str.), 1626 (aromatic C=N), 1548, 1437 (aromatic C=C), 1319, 1282, 1215, 1089, 962, 881, 773, 698, 545 and 451 cm-1 Analysis: Calculated for C16H8Cl2N6O2S2: C, 42.58, H, 1.79, Cl, 15.70, N, 18.62, S, 14.21. Found: C, 42.67, H, 1.92, Cl, 15.73, N, 18.60, S, 14.26.
Synthesis of 6,13-dichloro-3-methyl-9-methoxy-1-hydroxy-2,4,8-triazatriphenodithiazine (6)
Mixture of 3-amino-6-methoxypyridine-2-thiol (2 g, 12 mmol), 4-amino-2-methyl-6-hydroxypyrimidine-5-thiol (2 g; 10 mmol) was placed in a reaction flask equipped with magnetic stirrer, thermometer and reflux condenser. Absolute ethanol (120 mL) and 10 mL of 15 % HCl were then added and solution was warmed to dissolve. Tetrachloro-1,4-benzoquinone (3 g, 12 mmol) was later added and the mixture refluxed with continuous stirring for 7 h at 78 oC. At the end of the reflux period, the mixture was poured into a clean beaker and 100 mL of water was added, it was heated for 15 min and cooled. The crude product was collected by filtration, dried in an oven and crystallized from acetone-methanol mixture to give 6 as purple powder (4.79 g, 86 % yield). m.p > 300 oC; Uv-V (MeOH) λmax(nm) log( : 750 (1.5670), 583 (2.1691), 343 (2.6682), 257 (3.0207), 228 (2.3856), 223 (1.2553); IR (KBr) : 3751, 3346 (OH), 3119 (aromatic C-H-stretch), 2918, 2852 (C-H stretch of CH3, CH2) 1641, 1595 (aromatic C=N), 1546, 1444 (aromatic C=C) 1354, 1273, 1155, 1085, 1012, 912, 833, 777, 723, 677, 608, 553, and 509cm-1; 1H-NMR (DMSO-d6) δ: 11.64 (1H, s), 7.01 (2H, d, J = 144.25 Hz Het. Ar-H), 2.4 (3H, s, -OCH3), 1.45 (3H, s, -CH3); 13C-NMR (DMSO) δ: 165.96, 162.37, 159.79 (Ar-C), 88.53 (C=C), 21.43 (-CH3, aliphatic carbon); Analysis: Calculated for C17H9Cl2N5O2S2: C, 45.34, H, 2.01, Cl, 15.75, N, 15.55, S, 14.24.Found: C, 45.42, H, 2.30, Cl, 15.86, N, 15.53, S, 14.33.
Results and Discussion
2,3-Dichloro-1,4-naphthoquinone was refluxed with 2-aminothiophenol to yield 6-chloro-5H– benzo[a]phenothiazin-5-one 1. Compound 1 on treatment with 2,4-diamino-6-hydroxypyrimidine-5-thiol , obtained from alkaline hydrolysis of 2,4-diamino-6-hydroxy-5-thiacyanatopyrimidine, in the presence of anhydrous Na2CO3 under reflux yielded 7-amino-9-hydroxy-6,8-diazabenzo[a][1,4]benzothiazino[3,2-c]phenothiazine 2. The second derivative 7,14-diethylthio-9,12-dihydroxy-6,8,13,15-tetrazabenzo[a][1,4]benzothiazino[3,2-c]phenothiazine 3 was synthesized as a blue powder by condensation of 2 moles of 4-amino-2-ethylthio-6-hydroxypyrimidine-5-thiol with a mole of 2,3-dichloro-1,4-naphthoquinone in an acid medium. 3-Amino-6-methoxypyridine-2-thiol also condensed with the 2,3-dichloro-1,4-naphthoquinone in the presence of 15 % HCl and Ethanol to furnished a good yield of 8,13-dimethoxy-9,12-diazabenzo[a][1,4]benzothiazino[3,2-c]phenothiazine 4 as a brown crystalline powder (Scheme 1)
However, condensation of 2 moles of the 4-amino-2-ethylthio-6-hydroxypyrimidine-5-thiol with 1 mole of tetrachloro-1,4-benzoquinone in an acid medium gave 6,13-dichloro-3,10-diethylthio-1,8-dihydroxy-2,4,9,11-tetraazatriphenodithiazine 5 as greenish yellow powder. Another complex azatriphenodithiazine ring system (6,13-dichloro-3-methyl-9-methoxy-1-hydroxy-2,4,8-triazatriphenodithiazine) 6 was synthesized as purple crystalline powder by condensing 3-amino-6-methoxypyridine-2-thiol, tetrachloro-1,4-benzoquinone and 4-amino-2-methyl-6-hydroxy-5-thiopyrimidine Scheme 2.
The IR spectra of compound 1 showed >NH stretching vibration as weak band in the region 3125- 3063 cm-1 and the >C=O as strong band at 1631 cm-1. Bands at 1502cm-1 and 1608 cm-1 were assigned to C=C and C=N stretching vibrations in both compound 1 and 2 respectively. The disappearance of a band due to C=O and the appearance of new band due to C=N at 1608 cm-1 coupled with a shift in the absorption maxima from 491 nm in compound 1 to 780 nm in compound 2, because of double bond extension, indicated the formation of 2 from 1. The formations of 3 and 4 were also implicated by the appearance of bands 1556-1626 cm-1 and 1587 cm-1 ( C=N), 561-648 cm-1 and 648-692 cm-1 (C-S-C) for compounds 3 and 4 respectively and disappearance of the band for the –NH2 group in the precursor. Furthermore, the bands at 3375 cm-1 (OH), 3196 cm-1 (C-H str. aromatic), 1626 (aromatic C=N), 1548-1437 cm-1 (aromatic C=C) and 698, 545 (C-S-C in the thiazine ring) were in agreement with the assigned structure of compound 5 likewise those at 3751-3346 cm-1 (OH), 3119 (aromatic C-H str), 2918-2852 cm-1(C-H str of CH2, CH3), 1641- 1595 cm-1 (aromatic C=N), 1546-1444 cm-1 (aromatic C=C), 912-833 cm-1 (CH-bend in substd benzene ring), 777 cm-1 (C-Cl) and 677-609 cm-1 (C-S-C in the thiazine ring) were consistent with the structure of compound 6.
In the 1H-NMR spectra of compounds 2- 4, the multiplet due to aromatic protons appeared in the region ẟ 8.87- 7.91. The protons of the methine group and methyl group in –SCH2CH3 appeared at ẟ 3.30 and 3.06 respectively in compound 3. The peaks due to the four protons in pyridine rings of compounds 4 and 6 occurred between ẟ 7.66-7.01. The –OCH3 protons in these two compounds showed a singlet in the region ẟ 2.40 – 4.28 likewise the –CH3 protons in compound 6 at ẟ 1.45. The structural confirmation of the synthesized compounds was achieved with elemental analysis. With the exception of compounds 4 and 5 which showed few peaks due to the insolubility, the 13C-NMR of others led credence to the establishment of their structures.
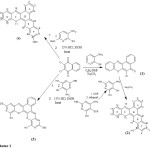 |
Scheme 1 Click here to View scheme |
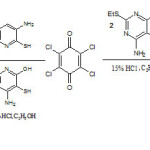 |
Scheme 2 Click here to View scheme |
Antimicrobial Activity
All the synthesized compounds were screened for their antimicrobial activity at concentration 20 mg/disc in agar media following the method of Bauer et al.32 Using Ciprofloxacin, an antibacterial and Ketoconazole, an antifungal activity as reference drugs, the compounds were screened against eight (8) micro-organisms, viz: Bacillus subtitis, Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumonia, Candida albican and Aspergillus niger. This was carried under sensitivity test and minimium inhibitory concentration (MIC).
Sensitivity Test
This assay was conducted by applying agar-well diffusion method33 using a concentration of 20 mg/mL of each compound. From the result (Table 1), the compounds showed significant activity against the test organisms except E. coli which was only sensitive to compounds 4 and 5. Bacillus cereus was sensitive to all the compounds. S.aureus, P. aeruginosa and K. pneumonia were resistant to compounds 4 and 5, but were sensitive to other compounds.
Minimum Inhibitory Concentration (MIC) Determination
This was carried out using agar dilution following the procedure outlined by chemical laboratory Standards Institute (CLSI) 34 using the following serial dilutions1, 0.5, 0.25, 0.125 and 0.0625. Almost all the synthesized phenothiazine derivatives were active against the microorganisms even at very low concentrations following from the fact that the lower the MIC values, the higher the activity. Compound 3 has the highest MIC values against bacteria which ranged from 0.0398- 0.1585 mg/mL (Table 3). There was no MIC for compound 4 and 5 against S. aureus and P. aeruginosa respectively likewise for compounds 1, 2 & 3 against E. coli which indicated that the listed micro-organisms were highly resistant to the respective compounds. The entire compounds were very active against B.cereus and B.substilis at lower MIC values. All the compounds were highly active against the fungi with an exception of C. albican which showed resistance to compounds 1 and 5.All the compounds were very active against the fungi, A. niger except compound 6. The reference drugs used were all active against the microorganisms with MIC lower than the synthesized compounds except in A.niger (see table 3).
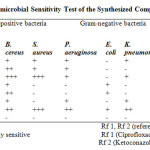 |
Table1: Results of Antimicrobial Sensitivity Test of the Synthesized Compounds Click here to View table |
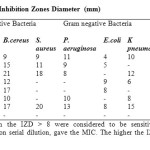 |
Table2: Results of the Inhibition Zones Diameter (mm) Click here to View table |
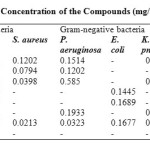 |
Table3: Minimum Inhibitory Concentration of the Compounds (mg/ml) Click here to View table |
References
- Sharma, R.; Samadhiya, P.; Srivastava, S. D.; Srivastava, S .K.; Synthesis and biological activity of 2-oxo-azetidine derivatives of phenothiazin,. Org Commun, 2011, 4(2), 42-51
- Khanna, R.; Palit, G.; Srivastava, V. K.; Shanker, K.; Newer Heterocycles of Phenothiazine and their Antiparkinsonian Activity, Indian J Chem , 29B , 1990, 556-560.
- Meghasham, N.N., Mahesh, K.G; Pravin, K.G.; Microwave Mediated Synthesis Of Pharmacological Active Substituted Derivatives Of 2-(4/-Phenothiazinyl Pyrazolyl) Pyrroles. World Journ of Pharm Res, 2014, 3(4), 1064-1073.
- Ei-Said, M.K. Pharmazie, 1981, 36, 678
- Tilak, S.R; Tyagi, R.; Goel, B.; Saxena, K.K.; Synthesis and Anti-Inflammatory Activity of Some Potential Cyclic Phenothiazines, Indian Drugs. 1998, 35:221
- Sadanandam, Y.S; Shetty, M.M; Rao, A. B.; Rambabu Y,10H-Phenothiazines: A New Class of Enzyme Inhibitors for Inflammatory Diseases, Eur J Med Chem, 2009, 44, 197-202
- Rajasekaran, A.; Thampi, P. P.; Synthesis and anti-inflammatory activity of some 10-[(1-acyl-1htetrazol-5-yl)ethyl-10-phenothiazine, Acta Pharm. Turcica, 2003, 45, 227-232
- Dominguez, J. N.; Lopez, S.; Charris, J.; Iarruso, L.; Lobo, G.; Semenow, A.; Olson, J. E.; Rosenthal, P .J.;, Synthesis and Antimalarial Effects of Phenothaizine Inhibitors of Plasmodium Falciparium Cysteine Protease, J Med Chem, 1997, 40, 2726-2732.
- Kalkanidis, M; Klonis, N.; Tilley, L.; Deady L.W.; Novel Phenothiazine Antimalarial: Synthesis, Antimalarial Activities and Inhibition of the Formation of Beta-haematin. Biochem Pharmacol, 2002, 63 (5), 833.
- Lin, G.; Medha, K.K; Synthesis of the Piperidinone Metabolites of Piperidine-type Phenothiazine Antipsychotic Drugs via Ruthenium Tetraoxide Oxidation, J Heterocycl Chem, 1991, 28, 215
- Mohamed, F. A.; Mohamed, H. A.; Hussein, S. A.; Ahmed, S.A.; A Validated Spectrofluorimetric Method for Determination of some Psychoactive Drugs, J Pharm Biomed Analysis, 2005, 39, 139–146.
- Wen, B.; Zhou, M.; Metabolic activation of the phenothiazine antipsychotics chlorpromazine and thioridazine to electrophilic iminoquinone species in human liver microsomes and recombinant P450s, Chemico-Biol Interact, 2009, 181, 220–226
- Raval, J.; Desai, K. K; Synthesis and Antimicrobial Activity of New Triazolopyridinyl Phenothiazines. ARKIVOC, 2005, 8, 21.
- Srivastava, S. K.; Dua, R.; , Srivastava S D, Synthesis and antimicrobial activity of [N1-(N-substitutedarylidene-hydrazino)-acetyl]-2-methyl-imidazoles and [N1-(4-substituted aryl-3-chloro-2-oxo-1-azetidinyl-amino)-acetyl]-2-methyl-imidazoles, Proc. Nat. Acad. Sci. India, Sec A, Phys Sci, 2010, 80,117-121.
- Trivedi , P .B.; Undavia, N .K.; Dave, A. M.; Bhatt, K. N.; Desai, N. C.; Synthesis and antimicrobial activity of 4-oxothiazolidines, 4-oxoazetidines, malonanilic acid hydrazines and pyrazoline derivatives of phenothiazine, Indian J Chem, 1993, 32B (7), 760-765.
- Viveros, M.; Amaral, L.; Design, Synthesis, Characterisation and Antitubarcular Activity of some 2-Heterocycle-Substituted Phenothiazines, Int J Antimicrob Ag, 2001, 17, 225.
- Amaral, L.; Kristiansen; Phenothiazines: an Alternative to Conventional Therapy for the Initial Management of Suspected Multidrug Resistant Tubercular Activity of some 2-Heterocycle-Substituted Phenothiazines, Int J Antimicrob Ag, 2001, 17, 225.
- Madrid, P. B.; Polgar, W. E.; Toll, L.; Tanga, M. J; Synthesis and antitubercular activity of phenothiazines with reduced binding to dopamine and serotonin receptors, Bioorg Med Chem Let, 2007, 17, 3014–3017.
- Motohasho, N.; Kawase, M.; Saito, S.; Sakagami, H.; Antitumor Potential and Possible Targets of Phenothiazine-Related Compounds, Curr Drug Targets, 2000, 1, 237-245.
- Motohasho, N.; Kawase, M.; Saito, S.; kurihara, T.; Satoh, K.; Nakashima, H.; Premanathan, M.; Arakaki, R.; Sakagami, H.; Molnar, J.; Synthesis and Biological Activity of N-acylphenothiazines, Int J Antimicrob Ag, 2000, 14(3), 203-207.
- Leancer D. and Mitscher L .A., Organic Chemistry of Drug Synthesis, Vol. I.; John Wiley & and Sons, New York, (1977), 372-392
- Kubota, K.; Kurebayashi, H.; Miyachi, H.; Tobe, M.; Onishi, M.; Isobe, Y.; Synthesis and Structure-activity Relationships of Phenothiazine Carboxylic acids having Pyrimidine-dione as Novel Histamine H(1) Antagonists, Bioorg Med Chem Lett, 2009, 19(10), 2766-2771.
- Borbely, A. A; Hinkkanen, M. L.; Synthesis and Antifungal Activity of some Substituted Phenothiazines and Related Compounds, Mod Pharmacol Toxicol, 1979, 16, 403-426.
- Korth, L .H.; Acridine and Phenothiazine Derivatives as Pharmacotherapeutics for Prion Disease, National Academy of Science USA, 2001, 98 (17), 9836-9841.
- Okafor, C. O.; Okerelu, I. O.; Okeke, S. I.; Vat Dyes from Three New Heterocyclic Ring, Dyes and Pigments, 1986, 8, 11-24.
- Pathak, V. N.; Yadav, S .S.; Srivastava, R .C.; Indian Sci. Abstr., 1994, 30, 17.
- Mitchell, S. C.; Mammallian Metabolism of Administered Phenothiazine: Drug Metabolism Rev, 1982, 13: 319-343.
- Murphy, C .M.; Rarner, H.; Smith, N .L.; Ind. Eng. Chem., 1950, 42, 2479
- Floyd, R. A.; Scheider, J. E.; Zhu, Y. Q.; North, T. W.; Schinazi, F.; Proc Am Assoc Cancer Res, 1993, 34, 359.
- Kurihara, T.; Motohashi, N.; Sakagami, H.; Molnar, H.; Relationship between Cytotoxin Activity and Dipole Moment for Phthalimido- and Chloroethyl-phenothiazines, J Anticancer Res, 1999, 19, 4081.
- Kurihara, T.;, Nojima, K.; Sakagami, H.; Molnar, H.; Motohashi, N.; Molnar, H.; Electronic Structure and Cytoxin Activity of ‘’half-mustard type’’ Phenothiazines by MM3 and PM3 Methods, J Anticancer Res, 1999, 19, 3895.
- Bauer, A. W; Kibby, W. M. M.; Sherris, J.C.; Turck, M.; Am J Clin Path, 1999, 45, 37.
- Perez, C.; Pauli, M.; Bazerque, P.; Antibiotic assay by the Agar-well Diffusion Method-Acta Biologiae et Medicine Experimentalis,1990, 15, 113-115.
- Fries, K.; Ochwat, P.; Beru. Chem. Neues uber, 1923, 56B,17,3334

This work is licensed under a Creative Commons Attribution 4.0 International License.









