Synthesis, Mechanism and Kinetic Studies of Cobalt(II) Schiff base Complexes with Organotin(IV)chlorides
Fatemeh Mosalanezhad1*, Reza Mosalanezhad2
1Department of Chemistry, Jahrom Branch, Islamic Azad University, Jahrom, Iran
2Department of Electrical Engineering, Jahrom Branch, Islamic Azad University, Jahrom, Iran
DOI : http://dx.doi.org/10.13005/ojc/300418
Article Received on :
Article Accepted on :
Article Published : 11 Dec 2014
The kinetics and mechanism of the adduct formation of diorganotin(IV)dichlorides (R2SnCl2) where R=Ph, Me, Bu and triphenyltin(IV)chloride with Co(II) tetraaza Schiff base complexes such as: [Co(ampen)] {[N,N'-ethylenebis-(o-amino-α-phenylbenzylideneiminato)cobalt(ΙΙ)]}, [Co(campen)] {[N,N'-ethylenebis-(5-chloro-o-amino-α-phenylbenzylideneiminato)cobalt(II)]} and [Co(amaen)] {[N,N'-ethylenebis-(o-amino-α-methylbenzylideneiminato)cobalt(ΙΙ)]}, [Co(appn)]{[N,N'-1,2-propylenebis-(o-amino-α-phenylbenzylideneiminato)cobalt(ΙΙ)]}, [Co(cappn)]{[N,N'-1,2-proylenebis-(5-chloro-o-amino-α-phenylbenzylideneiminato-)cobalt(II)]} were studied spectrophotometrically. The kinetic parameters and the rate constant values show the acceptor tendency trend for the organotin(IV)chlorides as follow: Ph2SnCl2 > Me2SnCl2 > Bu2SnCl2> Ph3SnCl Adducts have been separately synthesized and fully characterized by 119SnNMR, IR, Uv-Vis spectra and elemental microanalysis (C.H.N) methods. The trend of the rate constants for the adduct formation of the cobalt complexes with a given tin acceptor decreases as follow: [Co(amaen)] > [Co(appn)] > [Co(ampen)] > [Co(cappn)] > [Co(campen)]. The linear plots of kobs vs. the molar concentration of the organotin(IV) chlorides, the high span of the second order rate costant k2 values and the large negative values of ΔS≠ and low ΔH≠ suggest an associative (A) mechanism for the acceptor-donor adduct formation. Also [Co(aptn)]{[N,N'-1,3-propylenebis-(o-amino-α-phenylbenzylideneiminato)cobalt(ΙΙ)] and [Co(captn)]{[N,N'-1,3-proylenebis-(5-chloro-o-amino-αphenylbenzylideneiminato)cobalt (II)]} were synthesized and characterized but their kinetics with R2SnCl2 were very fast that we were unable to follow them using the conventional methods.
KEYWORDS:Diorganotin(IV)dichlorides; Kinetic; Mechanism; Tetraaza Schiff base; Cobalt(II) complex Triphenyltin(IV)chloride
Download this article as:| Copy the following to cite this article: Mosalanezhad F, Mosalanezhad R. Synthesis, Mechanism and Kinetic Studies of Cobalt(II) Schiff base Complexes with Organotin(IV)chlorides. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Mosalanezhad F, Mosalanezhad R. Synthesis, Mechanism and Kinetic Studies of Cobalt(II) Schiff base Complexes with Organotin(IV)chlorides. Available from: http://www.orientjchem.org/?p=5725 |
Introduction
Schiff bases form stable complexes with metals that perform an important role in biological systems [1]. Transition metal Schiff base complexes have many applications in the area of antitumoral, antiviral and antibacterial activity [2] and are also used as mimetic systems for enzyme models [3].
The tetraorganotin compounds show no evidence of Lewis acidity, the tin(IV) halides are strong Lewis acids which can produce 1:1 and 1:2 adducts in the presence of Lewis bases. The organotin halides RnSnX4-n are intermediate in Lewis acidity and found between R4Sn and SnX4. Generally the acidity is increased when the proportion of the halide is increased.
It is worth to note that till now no studies have been done on the kinetics and mechanism of the adduct formation between Co(II) tetraaza Schiff base complexes and the diorganotin(IV)dichlorides, although several studies have been done in our group on the thermodynamics of their adduct formation [4,5].
In this paper we have investigated the kinetics and mechanism of the interaction between the cobalt(II) tetraaza Schiff base complexes as donor and the organotin(IV)chlorides as acceptor. Cobalt(II) tetraaza Schiff base complexes such as [Co(ampen)], [Co(campen)], [Co(amaen)], [Co(cappn)] and [Co(appn)] were synthesized and characterized. The kinetics and mechanism of their adduct formation with diorganotin(IV)dichlorides, such as Me2SnCl2, Bu2SnCl2, Ph2SnCl2 and Ph3SnCl as acceptors in DMF solvent were studied spectrophotometrically, and explained by an associative (A) mechanism.
Experimental
Reagents
1,2-ethylenediamine, 2-amino-5-chlorobenzophenone, 2-amino-benzophenone, 2-amino- acetophenone, 1,2-propylenediamine, methanol, chloroform, cobalt(ΙΙ)acetatetetrahydrate, dimethyltin(IV)dichloride, dibutyltin(IV)dichloride, diphenyltin(IV)dichloride triphenyltin(IV)chloride and acetonitrile were commercially obtained from Merck , Fluka or Acros and used without further purification.
Instrument
Light-absorption measurements in the region (300-700 nm) were made with a Perkin-Elmer-Uv-Vis spectrophotometer-Lambda 2, equipped with a Lauda-ecoline-RE thermostat. FT-IR spectra were run on a Shimadzu FTIR-8300 spectrophotometer. 119SnNMR spectra were recorded on a Bruker Avance DPX-400 spectrometer in CDCl3 solvent. Elemental microanalyses (C.H.N.) were obtained using a Thermo Finnigan-CHNSO Analyzer.
Synthesis of Cobalt(II) Tetraaza Schiff Base Complexes
The four-coordinate complexes of N4 type, [Co(amaen)], [Co(ampen)], [Co(campen)], [Co(appn)], [Co(cappn)], [Co(aptn)] and [Co(captn)] were prepared according to the literature [6] (Fig. 1). The IR and the Uv-Vis absorption spectra were in good agreement with the published data [6].
![Fig1. The structure of [Co(amaen)] (1), [Co(ampen)] (2), [Co(campen)] (3), [Co(appn)] (4), [Co(cappn)] (5), [Co(aptn)] (6) and [Co(captn)] (7).](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_Fig1-150x150.jpg) |
Fig1: The structure of [Co(amaen)] (1), [Co(ampen)] (2), [Co(campen)] (3), [Co(appn)] (4), [Co(cappn)] (5), [Co(aptn)] (6) and [Co(captn)] (7). Click here to View figure |
Synthesis of Adducts of Diorganotin(IV)Dichlorides with Co(ΙΙ) Tetraaza Schiff Base Complexes
0.1 mmol R2SnCl2 was dissolved in 5 ml acetonitrile and dropwisely added to 0.1 mmol of the Co(ΙΙ) complexes in 10 ml acetonitrile. The mixture was stirred for 2h at room temperature. During that time the red color solution was changed to green precipitate. Changing in color is due to the interaction of R2SnCl2 with Co(ΙΙ) complexes. The precipitate was filtered off and dried under vacuum (Fig. 2).
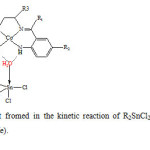 |
Fig2: The proposed structure of the adduct fromed in the kinetic reaction of R2SnCl2 with Co(ΙΙ) tetraaza Schiff base complexes (R=Me, Bu, Ph, R1=Me, Ph, R2=H, Cl,R3=Me). Click here to View figure |
Kinetic Measurement
A solution from each Co(ΙΙ) complex with certain concentration 6.4×10-5 M was prepared. In a typical measurement, 2.5 ml of this solution was transferred into the thermostated cell compartment of the Uv-Vis instrument, which was kept at constant temperature (runs from 10 – 40( ± 0.1ºC)) by circulating water. Excess concentration in the range (1×10-4– 4×10-2 M) of a given R2SnCl2 and (1.3×10-3– 1.1×10-1 M) of Ph3SnCl acceptor was added to this solution by Hamilton µL syringe.
The absorption measurements were carried out at various wavelengths where the different in absorptions were the maximum. The formed adduct shows absorption different from the donor, while the acceptors show no absorption at those wavelengths. The pseudo- first order rate constants kobs (s-1) were calculated by fitting the data to ln [(At – A∞)/(A0 – A∞)] = kobst (where At = absorbance at time t; A0 = absorbance at t = 0; A∞ = absorbance at t = ∞) by means of a linear least-squares computer program. The second-order rate constants k2 (M-1s-1) were obtained from the slope of the linear plots of kobs vs. [A] (acceptor concentration). The standard deviation values of the rate constants were obtained duplicate readings.
The activation parameters ΔH#, ΔS# were obtained from the linear Eyring plots of ln(k2/T) vs. 1/T at four different temperatures in the range (10-40ºC) using linear least squares computer program. Also the ΔG# values were computed by the same program using equation ΔG# =ΔH# -TΔS# at T=313K.
Results And Discussion
Spectral Characterization
Electronic Spectra
Absorption spectra of the Co(ΙΙ) complexes and their adducts with diorganotin(IV)dichlorides were examined over the range 300-700 nm, in DMF solvent and the results are summarized in (Table 1). In Co(ΙΙ) tetraaza Schiff base complex spectrum several intense absorption bands are observed in the Uv-Vis regions. By addition of R2SnCl2 to a solution of Co(ΙΙ) tetraaza Schiff base complex in DMF the original peaks of Co(ΙΙ) tetraaza Schiff base complex are changed. The electronic spectra of adducts formed at the end of the kinetic runs were the same as the electronic spectra of the respective separately synthesized adducts (Fig.3).
Table 1. The Uv-Vis bands λmax (nm) of the cobalt(ΙΙ) tetraaza complexes and their adducts with diorganotin(IV)dichlorides in DMF.
| Complex | λ (nm) |
| [Co(amaen)] | 371, 428, 540 |
| [Co(amaen)].(Ph2SnCl2).H2O | 366 (sh), 426 |
| [Co(amaen)].(Me2SnCl2).H2O | 427 (sh) |
| [Co(amaen)].(Bu2SnCl2).H2O | 361 (sh), 423 (sh) |
| [Co(campen)] | 387, 442, 552 |
| [Co(campen)].(Ph2SnCl2).H2O | 384, 440, 554 |
| [Co(campen)].(Me2SnCl2).H2O | 381, 439, 551 |
| [Co(campen)].(Bu2SnCl2).H2O | 380, 439, 551 |
| [Co(ampen)] | 377, 436, 542 |
| [Co(ampen)].(Ph2SnCl2).H2O | 369 (sh), 431 |
| [Co(ampen)].(Me2SnCl2).H2O | 431 (sh) |
| [Co(ampen)].(Bu2SnCl2).H2O | 370 (sh), 431 |
| [Co(appn)] | 479, 438, 541 |
| [Co(appn)].(Ph2SnCl2).H2O | 470 (sh), 432, 539 (sh) |
| [Co(appn)].(Me2SnCl2).H2O | 373 (sh), 432, 538 (sh) |
| [Co(appn)].(Bu2SnCl2).H2O | 369 (sh), 431, 536 (sh) |
| [Co(cappn)] | 387, 442, 554 |
| [Co(cappn)].(Ph2SnCl2).H2O | 384, 440, 553 |
| [Co(cappn)].(Me2SnCl2).H2O | 370 (sh), 440, 552 (sh) |
| [Co(cappn)].(Bu2SnCl2).H2O | 384, 439, 551 |
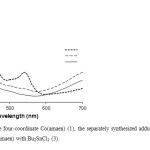 |
Fig3: The electronic spectra of the four-coordinate Co(amaen) (1), the separately synthesized adduct of Bu2SnCl2 with Co(amaen) (2) and the end of the kinetics of Co(amaen) with Bu2SnCl2 (3). Click here to View figure |
119SnNMR Spectra
Holeĉek and coworkers studies show that the di- and tri-organotin 119SnNMR spectra can be used as an indicator of the coordination number of the tin atom. In the ranges of +200 to -60, -90 to -190, -210 to -400, -440 to -540 ppm, the coordination number of the tin atom is four, five, six and seven, respectively [7-10]. The adducts formed in the present investigation exhibit the 119Sn spectra in the range -110 to -182 ppm , suggesting that the tin atom is five-coordinated (Table 2).
Table 2. 119SnNMR characterization of some cobalt(ΙΙ) adducts with diorganotindichlorides
|
119Sn(δ,ppm) |
Complex |
|
-182.42 |
[Co(amaen)].(Ph2SnCl2).H2O |
|
-176.35 |
[Co(amaen)].(Me2SnCl2).H2O |
|
-168.33 |
[Co(amaen)].(Bu2SnCl2).H2O |
|
-110.12 |
[Co(campen)].(Ph2SnCl2).H2O |
|
-120.39 |
[Co(campen)].(Me2SnCl2).H2O |
|
-145.65 |
[Co(campen)].(Bu2SnCl2).H2O |
|
-165.54 |
[Co(ampen)].(Ph2SnCl2).H2O |
|
-143.25 |
[Co(ampen)].(Me2SnCl2).H2O |
|
-151.18 |
[Co(ampen)].(Bu2SnCl2).H2O |
|
-1889.0 |
[Co(appn)].(Ph2SnCl2).H2O |
|
-166.0 |
[Co(appn)].(Me2SnCl2).H2O |
|
-179.0 |
[Co(appn)].(Bu2SnCl2).H2O |
|
-142.0 |
[Co(cappn)].(Ph2SnCl2).H2O |
|
-120.0 |
[Co(cappn)].(Me2SnCl2).H2O |
|
-166.0 |
[Co(cappn)].(Bu2SnCl2).H2O |
IR Spectra
The stretching vibration of the azomethine group (C=N) is observed in the range (1610-1680 cm-1). The stronger bands in the range (1440-1600 cm-1) are due to the skeleton stretching vibration of C=C of the benzene ring. The band at about 400 cm-1 can be assigned to the (Co-N) vibration. This band is the characteristic band of the complexes and was not found in the free Schiff base ligand spectra. The results show that, by formation of adducts, the C=N vibrations are shifted toward higher frequencies about (13-25 cm-1). The ring skeletal vibrations (C=C) are weakly affected by complexation. By formation of adducts, the (N-H) vibrations have been shifted toward higher frequencies. In the IR spectra of adducts, vibration bands in the range (3400-3450 cm-1) as well as at (704-725 cm-1) is assigned to (O-H) stretching of the coordinated H2O present [11] in adduct formed (Table 3).
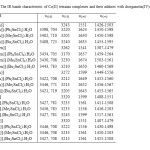 |
Table3: The IR bands characteristic of Co(ΙΙ) tetraaza complexes and their adducts with diorganotin(IV)dichlorides. Click here to View table |
Elemental Microanalysis 
Elemental analysis of the synthesized products have good agreement with the proposed adducts of the kinetic runs with the stochiometric composition 1:1 for Co(ΙΙ) tetraaza: R2SnCl2 with one coordinated H2O molecule (Table 4).
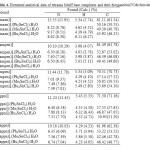 |
Table4: Elemental analytical data of tetraaza Schiff base complexes and their diorganoltin(IV)dichloride adducts. Click here to View table |
Kinetic Studies
Tables (5-24) show the rate constants and the activation parameters for the kinetic interaction between diorganotin(IV)dichlorides and triphenyltin(IV)chloride with the cobalt(II) tetraaza Schiff base complexes in DMF as solvent at various temperatures. The kinetics were followed under pseudo-first-order conditions for the acceptor concentration; the donor concentration was kept constant at 6.4×10-5 M, and the excess concentration of each acceptor was varied in the range 1×10-4– 4×10-2 M. The results in Tables (5-24) show that there is a linear rate dependence on the concentration of the acceptor [A].
![Table 5 Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(campen) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T5-150x150.jpg) |
Table5: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(campen) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 6. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(campen) with Me2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T6-150x150.jpg) |
Table6: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(campen) with Me2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 7. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(campen) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T7-150x150.jpg) |
Table7: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(campen) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 8. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(ampen) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T8-150x150.jpg) |
Table8: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(ampen) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 9. Pseudo -First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(ampen) with Me2SnCl2 in DMF at Different TTemperatTemperature. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T9-150x150.jpg) |
Table9: Pseudo -First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(ampen) with Me2SnCl2 in DMF at Different TTemperatTemperature. [Complex]= 6.4×10-5M Click here to View table |
![Table 10. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(ampen) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T10-150x150.jpg) |
Table10: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(ampen) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 11. Pseudo-First-Order Rate Constants 103kobsa(s-1) for the Reaction of Co(amaen) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T11-150x150.jpg) |
Table11: Pseudo-First-Order Rate Constants 103kobsa(s-1) for the Reaction of Co(amaen) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 12. Pseudo-First-Order Rate Constants 103kobsa(s-1) for the Reaction of Co(amaen) with Me2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T12-150x150.jpg) |
Table12: Pseudo-First-Order Rate Constants 103kobsa(s-1) for the Reaction of Co(amaen) with Me2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 13. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(amaen) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T13-150x150.jpg) |
Table13: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(amaen) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 14. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(cappn) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T14-150x150.jpg) |
Table14: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(cappn) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 15. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(cappn) with Me2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T15-150x150.jpg) |
Table15: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(cappn) with Me2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 16. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(cappn) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T16-150x150.jpg) |
Table16: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(cappn) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 17. Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(appn) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T17-150x150.jpg) |
Table17: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(appn) with Ph2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![IX Table 18. Pseudo -First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(appn) with Me2SnCl2 in DMF at Different TTemperatTemperature. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T18-150x150.jpg) |
Table18: Pseudo -First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(appn) with Me2SnCl2 in DMF at Different TTemperat Temperature. [Complex]= 6.4×10-5M Click here to View table |
Since most of the kinetic studies [12] that have been published till now were carried out in interfering (i.e. coordinating) solvents, it is necessary to investigate the nature of this interference before the mechanistic assignment is secure [13]. Plots of kobs vs. R2SnCl2 and plots of kobs vs. Ph3SnCl exhibit an almost zero intercept. The present studies indicate that solvolysis of Co(II) tetraaza in DMF is negligible. Typical plots of kobs vs. Ph2SnCl2 molar concentrations [A] for [Co(ampen)] at different temperatures are shown in Fig.4.
![Fig4. The plots of kobs vs. Ph2SnCl2 molar concentrations [A] for [Co(ampen)] at different temperatures.](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_Fig4-150x150.jpg) |
Fig4: The plots of kobs vs. Ph2SnCl2 molar concentrations [A] for [Co(ampen)] at different temperatures. Click here to View figure |
![Table 19 Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(appn) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T19-150x150.jpg) |
Table19: Pseudo-First-Order Rate Constants 103kobsa (s-1) for the Reaction of Co(appn) with Bu2SnCl2 in DMF at Different Temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 20. Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(amaen) with Ph3SnCl in DMF at different temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T20-150x150.jpg) |
Table20: Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(amaen) with Ph3SnCl in DMF at different temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 21. Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(appn) with Ph3SnCl in DMF at different temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T21-150x150.jpg) |
Table21: Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(appn) with Ph3SnCl in DMF at different temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 22. Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(ampen) with Ph3SnCl in DMF at different temperatures.[Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T22-150x150.jpg) |
Table22: Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(ampen) with Ph3SnCl in DMF at different temperatures.[Complex]= 6.4×10-5M Click here to View table |
![Table 23. Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(cappn) with Ph3SnCl in DMF at different temperatures. [Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T23-150x150.jpg) |
Table23: Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(cappn) with Ph3SnCl in DMF at different temperatures. [Complex]= 6.4×10-5M Click here to View table |
![Table 24. Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(campen) with Ph3SnCl in DMF at different temperatures.[Complex]= 6.4×10-5M](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_T24-150x150.jpg) |
Table24: Pseudo-first-order rate constants 103kobsa (s-1) for the reaction of Co(campen) with Ph3SnCl in DMF at different temperatures.[Complex]= 6.4×10-5M Click here to View table |
 |
Table25: Activation Parametersa ∆G#, ∆H# and ∆S# for the Reaction of Cobalt(ΙΙ) Complexes with Diorganotindichlorides in DMF Click here to View table |
The isosbestic points observed for the reaction of R2SnCl2 with [Co(campen)] were at (588, 354 nm), with [Co(ampen)] were at (576, 343 nm) and with [Co(amaen)] were at (574, 343 nm). As an example, the variation of the electronic spectra for [Co(amaen)], reacted with excess Ph2SnCl2 at 30ºC in DMF is shown in Fig. 5. The isosbestic points show that there is only one reaction in progress. The same procedure was followed for other systems and similar results were observed.
![Fig5. The absorption spectra of the kinetics of [Co(amaen)], with twelve fold excess Ph2SnCl2 at 30ºC in DMF with the isosbestic points.](http://www.orientjchem.org/wp-content/uploads/2014/12/Vol30_No4_Synth_Fatem_Fig5-150x150.jpg) |
Fig5: The absorption spectra of the kinetics of [Co(amaen)], with twelve fold excess Ph2SnCl2 at 30ºC in DMF with the isosbestic points. Click here to View figure |
Adduct entities were obtained from the reaction of the acceptors with the donors, according to Eq. (1):
[Co(L)].H2O + R2SnCl2or Ph3SnCl→[Co (L)].(R2SnCl2).H2O or [Co (L)].(Ph3SnCl).H2O (1)
where L = campen, ampen, amaen, appn, cappn and R = Me, Bu, Ph.
The rate law of Eq. (2) is compatible with the adduct formation according to Eq. (1):
kobs= k2 [A] (2)
The activation parameters, DH# and DS# (Table 25)were calculated as a function of temperature by Eyring Eq. (3):
ln (k2/T)= -ΔH#/RT + ΔS#/R + 23.8 (3)
A typical linear Eyring plots of ln(k2/T) vs. 1/T at four different temperatures in the range (10-40ºC) for Co(ampen) donor with different acceptors is shown in Fig. 6. The Eyring plots for the reaction of different donors with the acceptor Ph3SnCl (Lewis acid) in DMF is shown in Fig. 7.The low ΔH# values and the large negative DS# values is compatible with (A) mechanism. Also the linear plots of kobs vs. R2SnCl2 and or Ph3SnCl the high span of k2 values differing with the nature of the acceptors suggest an associative (A) mechanism.
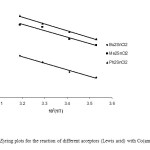 |
Fig6: The Eyring plots for the reaction of different acceptors (Lewis acid) with Co(ampen) in DMF. Click here to View figure |
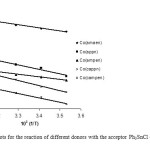 |
Fig7: The Eyring plots for the reaction of different donors with the acceptor Ph3SnCl (Lewis acid) in DMF. Click here to View figure |
The Acceptor Properties of the Diorganotin(IV)dichlorides and Triphenyltin(IV)chloride
Comparing the k2 values, the following trend of acidity for the acceptors used was assessed: Ph2SnCl2 > Me2SnCl2 > Bu2SnCl2 > Ph3SnCl.
It is clear that the electron withdrawing groups (Ph-) on the tin center makes Ph2SnCl2 a stronger Lewis acid than the others. This trend also indicates that replacing the methyl- by a more bulky butyl- group on the organotin(IV) compound causes a weakening of the interaction. The butyl- group can affect the interaction in two ways: 1) A more bulky butyl- group makes adduct formation unfavorable because of its greater steric hindrance than a methyl- group [14]. 2) Butyl- group, though, have better electron releasing properties to reduce the acid strength of the di-organotin(IV) Lewis acid but its steric effect would predominate and compensates the higher electronic effect. Although the electronic effect signifies that the k2 values must be higher with the triphenyltin(IV)chloride , but its steric effect is predominating (see Table 7) [15]. These are compitable with the electronegativity of Ph=2.717, and Cl=3.0 in Pauling and 3.475 in Sanderson’s scales [16].
Comparing the Donor Property of the Cobalt(II) Tetraaza Schiff Base Complexes
The k2 values for the ligands entry show high span, suggesting the dependence of rate on the nature of the ligand. The low ΔH# values and the large negative ΔS# values are compatible with (A) mechanism. Also the linear plots of kobs vs. [R2SnCl2], [Ph3SnCl] and the high span of k2 values differing with the nature of the acceptors suggest an associative (A) mechanism.
The obtained trend shows that, k2 values for reaction of [Co(campen)] is the least because of two electron withdrawing chlorides on the aromatic rings and two phenyl groups while [Co(amaen)] show the highest k2 values because of two electron donating methyl groups. It is clear that the existence of methyl groups on the imine group make the Schiff base complexes better donors. In [Co(ampen)], however there is two electron withdrawing phenyl groups on the imine group that makes it a weaker donor compared with [Co(amaen)]. In [Co(appn)] there is one methyl group on the ethylene group which makes it a better donor compared with [Co(ampen)]. The presence of two chloride groups on the aromatic rings makes it a weaker donor compared with [Co(appn)]. Therefore the second order rate constants k2 decrease according to the sequence:
[Co(amaen)] > [Co(appn)] > [Co(ampen)] > [Co(cappn)] > [Co(campen)].
Conclusions
The results of this work can be summarized as follow
- The relative Lewis acidities of different organotin(IV)chlorides toward a given Co(ΙΙ) tetraaza Schiff base complex according to the k2 values follow the trend: Ph2SnCl2 > Me2SnCl2 > Bu2SnCl2 > Ph3SnCl.
- The linear plots of kobs vs. [R2SnCl2], the high span of k2 values and the large negative DS# values suggest an associative (A) mechanism.
- The kinetic parameters and the second order rate constants k2 show the following trend of the donor properties of Co(ΙΙ) tetraaza Schiff base complexes toward a given acceptor:
[Co(amaen)] > [Co(appn)] > [Co(ampen)] > [Co(cappn)] > [Co(campen)].
Acknowledgement
We are grateful to Jahrom University Research Council for its financial support.
References
- C. P. Raptopoulou, A. N. Papadopoulos, D. A. Malamatari, E. Loannidis, G. Molsidis, A. Terzis, D. P. Kessissoglou, Inorg. Chim. Acta. 1998; 272, 283.
- R. Pignatello, A. Panicol, P. Mazzone, M. Pinizzotto, A. Garozzo, P. Furneri, J. Med. Chem. 1994, 29, 781.
- L. Guofa, S. Tongshun, Z. Yonghian, J. Mol. Struct. 1997; 412, 75.
- M. Asadi, K. Aein Jamshid, A. H. Kyanfar, J. Coord. Chem. 2008, 61, 1115.
- M. Asadi, K. Aein Jamshid, Transition. Met. Chem. 2007, 32, 822.
- M. Green, P. A. Tasker, J. Chem. Soc. A. 1970, 3105
- J. Hole ek, M. Nadvornik, K. Handlir, A. Lycka, J. Organomet. Chem. 1983, 241, 177.
- J. Hole ek, M. Nadvornik, K. Handlir, A. Lycka, J. Organomet. Chem. 1983, 258, 147.
- M. Nadvornik, J. Hole ek, K. Handlir, A. Lycka, J. Organomet. Chem. 1984, 275, 43.
- A. Lycka, J. Hole ek, M. Nadvornik, K. Handlir, J. Organomet. Chem. 1985, 280, 323.
- Abd El-Wahab, Z.H.; El-Sarrag, M. R. Spect Chim Acta A. 2004, 60, 271.
- M. L. Tobe, J. Burgess, Inorganic Reaction Mechanism; Addison-Wesley Longman, Harlow: England, 1999; Chapter 3.
- J. Burgess, A. Parsons, Polyhedron. 1993, 12, 1959.
- C. H Yoder, D. Mokrynk, J. N. Spencer, S. M Coly, R. E. Otler, A. Haines, L. rushow, J. rganometallics. 1987, 6, 1679 .
- M. Asadi, Z. Asadi, F. Mosalanezhad, Intern. J. Chem. Kinetics. 2010, 42, 499.
- March, J.; Smith, M. B. March’s Advanced Organic Chemistry, 5th edition, John Wiley & sons, 2001; 1(1), 14-15.

This work is licensed under a Creative Commons Attribution 4.0 International License.









