Synthesis, Characterization and Antimicrobial Activities of 1,2,4-Triazole /Isatin Schiff bases and their Mn(II), Co(II) complexes
Sunita Bajroliya, G. S. Kalwania*, Savita Choudhary and S. Chomal
Department of Chemistry, S. K. Government P. G. College Sikar-332001, Rajasthan, India
DOI : http://dx.doi.org/10.13005/ojc/300419
Article Received on :
Article Accepted on :
Article Published : 11 Dec 2014
Mn(II) and Co(II) metal complexes have been synthesized with newly synthesized Schiff bases derived from isatin/5-nitroisatin and 3-substituted-4-amino-5-mercapto-1,2,4-triazole by new environmental benign microwave irradiation method as well as conventional method. Reaction achieved by microwave irradiation technique, require drastically reduced reaction time and provide high yield with improved selectivity as compared to conventional method. The synthesized compounds were characterized by elemental analysis as well as spectral studies. The elemental analysis were clearly indicated that ML2 type complexes have 1:2 stoichiometry (M=metal, L=ligand). The synthesized Schiff bases and their metal complexes screened for antimicrobial activities against selected bacteria and fungi.
KEYWORDS:Schiff bases; 1; 2; 4-Triazole; Isatin; 5-Nitroisatin; Microwave irradiation; Antimicrobial Complexes
Download this article as:| Copy the following to cite this article: Bajroliya S, Kalwania G. S, Choudhary S, Chomal S. Synthesis, Characterization and Antimicrobial Activities of 1,2,4-Triazole /Isatin Schiff bases and their Mn(II), Co(II) complexes. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Bajroliya S, Kalwania G. S, Choudhary S, Chomal S. Synthesis, Characterization and Antimicrobial Activities of 1,2,4-Triazole /Isatin Schiff bases and their Mn(II), Co(II) complexes. Available from: http://www.orientjchem.org/?p=5711 |
Introduction
The chemistry of 1,2,4-triazole and its derivatives is particularly interesting because of their potential application in medicinal, agricultural and industrial fields1-4. Derivatives of 1,2,4-triazoles show variety of biological activities such as antimicrobial5 anticonvulsant6, anticancer7, analgesic8, anti HIV9, and anti-inflammatory10 properties. Similarly, Isatin and Schiff bases of isatin derivatives are reported to show diverse biological activities like antibacterial11, antifungal12, anticonvulsant13, anti-HIV14, antidepressant15 and anti-inflammatory16 activities. It is well known that the heterocyclic compounds containing both 1,2,4-triazole and isatin rings have broad spectrum of pharmacologic properties2-8,11-15, 17-18. Some metal complexes derived from isatin and 1,2,4-triazole Schiff bases have reported to exhibit antimicrobial activities19.
These diverse biological applications of schiff bases and their precursors have inspired us to synthesize a new series of [Mn(II), Co(II)] metal complexes with Schiff bases containing isatin/5-nitroisatin and 3-substituted-4-amino-5-mercapto-1,2,4-triazole.
In recent years Microwave assisted reactions have attracted researchers muchinterest because of simplicity of operation, enhanced reaction rates, high yields, improved selectivity20-21 and environmental friendly reaction condition22-24. The reactions, which are not possible under normal conditions,can be overcome by high-energy microwave irradiation.There for we have made efforts to develop and optimize anew environmentally benign method using microwave irradiation in our laboratory and finally 3-substituted-4-amino-5-mercapto-1,2,4-triazole25 and isatin derivatives have been synthesized. These compounds were also prepared by conventional methods26 to compare their results with new microwave irradiation method. The newly synthesized compounds were characterized by spectral as well as elemental analysis and screened for their biological activities.
Experimental
The melting point of synthesized compounds were determined on open aluminum block and not corrected. Purity was checked by TLC (thin layer of silica gel) using Merck Silica gel G-60. Infrared (IR) spectra of all the synthesized compounds recorded using KBr from Shimadzu FTIR, Affinity-1. The NMR spectra has taken by Varian Gemini 400 spectrometer (300MHz) using TMS as an internal standard. Elemental data obtained from all the synthesized compounds were found satisfactory.
Results and Discussion
The focus of the present work was on the synthesis of some new Schiff bases and their Mn (II), Co (II) metal complexes by microwave irradiation (Scheme 1). These compounds were also synthesized by conventional method26. In microwave method, the reaction time reduced from 6 hrs.to 3-5 minutes (3a-h) and from4 hrs.to 4-7 minutes (4a-p). The yield obtained from microwave method was better as compared to conventional method.The purity of the compounds was checked by TLC. The compounds were characterized with the help of spectral data (IR, 1H NMR) and elemental analysis. All data were found in accordance with the proposed structures of synthesized compounds. The IR spectroscopic data have indicated the formation of 3a-h by disappearance of the NH2 band at 3400 cm-1 (2a-d) and appearance of C=N band at about 1608-1643 cm-1, The stretching vibrational N-H band at about 3201-3271 cm-1 and C=O band at about 1707-1738 cm-1confirm the presence of in dole ring in compounds 3a-h. A characteristic strong band at 2690–2716 cm−1clearly indicate the presence of S-H group intriazole and another band around at 1098- 1112 cm−1 is assigned to ν(C=S)27. These observations indicate that the Schiff bases exhibit thiol–thionetautomerism.
 |
Scheme 1 Click here to View scheme |
 |
Table1: Physical properties and analytical data of metal complexes (4a-p). Click here to View table |
All the metal complexes exhibited the band of ν(C=N) in the region 1592–1630 cm−1, showing the shift of this band to lower wave numbers, as compared with the spectra of the Schiff bases. This shift indicates that the nitrogen is coordinated to the metal ion28-30. The band of ν(C=O) in the region 1702–1730 cm−1 in the complexes, showing the shift to lower wave numbers compared with Schiff bases confirms that the carbonyl oxygen is also coordinated to the metal ion 31-32. The new bands in the region of 460–472 cm−1 in the spectra of the metal complexes are assigned to stretching frequencies of (M–N) bonds. The absence of a band around at 2700 cm−1 in all the metal complexes and new band in the region 358–380 cm−1 in far IR-spectra indicate the formation of metal-sulfur bonds. All the infrared frequencies of metal complexes are summarized in Table 2.The comparison of 1H NMR spectroscopic data of Schiff bases and their precursors also indicate the formation of 3a-h by the appearance of signals corresponding of two different NH protons at about 11.10-12.50 ppm and the disappearance of the NH2 protons in the 2a-d at about 5.00 ppm.
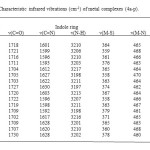 |
Table2: Characteristic infrared vibrations (cm-1) of metal complexes (4a-p). Click here to View table |
All the Schiff bases (3a-h) and their metal complexes (4a-p)along with some standard antibiotics and antifungal drugs were screened for their antimicrobial activities against selected bacteria and fungus. All the antimicrobial activity results are summarized in Table 3. The nitro derivatives of Schiff bases (3e-h) were found more active as compared to others. Co (II) complexes (4i-p) show higher antimicrobial activity in comparison to respective Schiff bases against selected bacteria and fungi.
 |
Table3: Antimicrobial activities of schiff bases and their metal complexes. Click here to View table |
General Method for the Synthesis of Schiff bases
All the Schiff bases were synthesized by both conventional and microwave assisted methods. The latter method provided reproducible results and analytical data related to the products obtained from both methods were found coherent.
Conventional Method
The equimolar quantity of 3-substituted-4-amino-5-mercapto-1,2,4-triazole (10 mmol) and isatin/5-nitroisatin (10 mmol) containing 3-4 drops concentrated HCl in hot methanol (40 ml) was refluxed for 6 hrs. After completion of reaction, the reaction mixture was cooled at room temperature. This solid obtained was filtered, washed with cold methanol, dried and recrystallized from methanol.
Microwave Assisted Method
A mixture of 3-substituted-4-amino-5-mercapto-1,2,4-triazole (1 m mol) and isatin/5-nitroisatin (1 mmol) in methanol ( 10 ml ) containing concentrated HCl (3-4 drops) was subjected to microwave irradiation intermittently at 30 seconds for 3-5 minutes. After completion of reaction as monitored by TLC, the reaction mixture was cooled at room temperature.The solid separated out on cooling was filtered, washed with cold methanol, dried and recrystallized from methanol.
The analytical data for all the synthesizes Schiff bases are summarized here. The yield obtained by microwave -assisted method is represented in bracket.
(3a). 3-[3-Phenyl-2’,4’-dihydro-1’,2’,4’-triazol-5’-mercapto-4’-yl]iminoisatin
Yield 72%, (95%); m.p. 221◦C; IR (KBr, νmax., cm-1): 1728 (>C=O), 1614 (C=N), 3211(N-H, isatin), 3187 (N-H, triazole), 2715 (S-H), 1104 (C=S); 1H-NMR (DMSO-d6, δ ppm): 6.96-7.80 (Ar-9H, m), 11.98 (isatin, N-H, 1H, s), 11.14 (triazole, N-H, 1H, s), 11.20 (S-H, 1H, s); Anal. Calculated (%) for C16H11N5OS ; C: 59.82, H: 3.41, N: 21.80; Found (%); C: 59.81, H: 3.40, N: 21.80.
(3b). 3-[3’-(2’’-Hydroxyphenyl)-2’,4’-dihydro-1’,2’,4’-triazol-5’-mercapto-4’-yl]iminoisatin
Yield 64%, (91%); m.p. 178◦C; IR (KBr, νmax., cm-1): 1730 (>C=O), 1613 (C=N), 3208(N-H, isatin), 3165 (N-H, triazole), 2706 (S-H), 1106 (C=S), 756 (1,2-disubstitutedphenyl); 1H-NMR (DMSO-d6, δ ppm): 6.93-7.78 (Ar-8H, m), 11.92 (isatin, N-H, 1H, s), 11.12 (triazole, N-H, 1H, s), 11.18 (S-H, 1H, s); Anal. Calculated (%) for C16H11N5O2S ; C: 56.97, H: 3.26, N: 20.78; Found (%); C: 56.97, H : 3.26, N: 20.77.
(3c). 3-[3’-(4’’-Hydroxyphenyl)-2’,4’-dihydro-1’,2’,4’-triazol-5’-mercapto-4’-yl]iminoisatin
Yield 66%, (92%); m.p. 249 ◦C; IR (KBr, νmax., cm-1): 1731 (>C=O), 1614 (C=N), 3209(N-H, isatin), 3168 (N-H, triazole), 2705 (S-H), 1105 (C=S), 841 (1,4-disubstitutedphenyl); 1H-NMR (DMSO-d6, δ ppm): 7.95-7.80 (Ar-8H, m), 11.91 (isatin, N-H, 1H, s), 11.13 (triazole, N-H, 1H, s), 10.85 (S-H, 1H, s); Anal. Calculated (%) for C16H11N5O2S ; C: 56.97, H: 3.27, N: 20.77; Found (%); C: 56.97, H : 3.26, N: 20.79.
(3d). 3-[3’-(4’’-Bromophenyl)-2’,4’-dihydro-1’,2’,4’-triazol-5’-mercapto-4’-yl]iminoisatin
Yield 62%, (88%); m.p. 260◦C; IR (KBr, νmax., cm-1): 1726 (>C=O), 1608 (C=N), 3201 (N-H, isatin), 3134 (N-H, triazole), 2690 (S-H), 1098 (C=S), 845 (1,4-disubstitutedphenyl); 1H-NMR (DMSO-d6, δ ppm): 7.08-7.90 (Ar-8H, m), 12.00 (isatin, N-H, 1H, s), 11.15 (triazole, N-H, 1H, s), 10.98 (S-H, 1H, s); Anal. Calculated (%) for C16H10N5OSBr ; C: 48.03, H: 2.50, N: 17.52; Found (%); C: 48.01, H : 2.50, N: 17.50.
(3e).3-[3’-Phenyl-2’,4’-dihydro-1’,2’,4’-triazol-5’-mercapto-4’-yl]imino-5-nitroisatin
Yield 70%, (90%); m.p. 269◦C; IR (KBr, νmax., cm-1): 1707 (>C=O), 1632 (C=N), 3265(N-H, isatin), 3121 (N-H, triazole), 2716 (S-H), 1106 (C=S), 1355 1541 (NO2); 1H-NMR (DMSO-d6, δ ppm): 7.12-8.40 (Ar-8H, m), 12.42 (isatin, N-H, 1H, s), 11.81 (triazole, N-H, 1H, s), 10.97 (S-H, 1H, s); Anal. Calculated (%) for C16H10N6O3S; C: 52.44, H: 2.73, N: 22.96; Found (%); C: 52.45, H: 2.73, N: 22.95.
(3f). 3-[3’-(2’’-Hydroxyphenyl)-2’,4’-dihydro-1’,2’,4’-triazol-5’-mercapto-4’yl]imino-5-nitroisatin
Yield 65%, (86%); m.p. 290◦C; IR (KBr, νmax., cm-1): 1714 (>C=O), 1641 (C=N), 3271(N-H, isatin), 3116 (N-H, triazole), 2710 (S-H), 1110 (C=S), 764 (1,2-disubstitutedphenyl), 1350 1542 (NO2); 1H-NMR (DMSO-d6, δ ppm): 7.39-8.16 (Ar-7H, m), 12.29 (isatin, N-H, 1H, s), 11.62 (triazole, N-H, 1H, s), 11.20 (S-H, 1H, s); Anal. Calculated (%) for C16H10N6O4S ; C: 50.27, H: 2.61, N: 21.97; Found (%); C: 50.26, H : 2.61, N: 21.98.
(3g).3-[3’-(4’’-Hydroxyphenyl)-2’,4’-dihydro-1’,2’,4’-triazol-5’-mercapto-4’-yl]imino-5-nitroisatin
Yield 67%, (94%); m.p. 280◦C; IR (KBr, νmax., cm-1): 1718 (>C=O), 1640 (C=N), 3268 (N-H, isatin), 3168 (N-H, triazole), 2708 (S-H), 1110 (C=S), 844 (1,4-disubstitutedphenyl), 1350 1542 (NO2); H-NMR (DMSO-d6, δ ppm): 7.35-8.18 (Ar-7H, m), 12.30 (isatin, N-H, 1H, s), 11.59 (triazole, N-H, 1H, s), 10.96 (S-H, 1H, s); Anal. Calculated (%) for C16H10N6O4S ; C: 50.27, H: 21.98, N: 20.77; Found (%); C: 50.27, H : 2.60, N: 21.99.
(3h).3-[3’-(4’’-Bromophenyl)-2’,4’-dihydro-1’,2’,4’-triazol-5’-mercapto-4’-yl]imino-5-nitroisatin
Yield 60%, (90%); m.p. 292◦C; IR (KBr, νmax., cm-1): 1738 (>C=O), 1643 (C=N), 3230 (N-H, isatin), 3130 (N-H, triazole), 2702 (S-H), 1112 (C=S), 852 (1,4-disubstitutedphenyl), 1362 1540 (NO2); 1H-NMR (DMSO-d6, δ ppm): 7.23-8.65 (Ar-7H, m), 12.49 (isatin, N-H, 1H, s), 11.96 (triazole, N-H, 1H, s), 10.90 (S-H, 1H, s); Anal. Calculated (%) for C16H9N6O3SBr ; C: 43.15, H: 2.04, N: 18.87; Found (%); C: 43.15, H : 2.02, N: 18.88.
General Method for the Synthesis of Metal Complexes
All the metal complexes were synthesized by both conventional and microwave assisted methods. The latter method provided reproducible results and analytical data related to the products obtained from both methods were found coherent.
Conventional Method
A solution of metal chloride (1 mmol)in methanol (6 ml) and 2 mmol of sodium acetate were added to Schiff base (2 mmol)dissolved in Methanol (30 ml) and refluxed on water bath for 4 hrs. The reaction mixture was cooled at room temperature and solid separated out was filtered, washed thoroughly with methanol and water and dried.
Microwave Assisted Method
A solution of metal chloride (1 mmol)in methanol (3 ml) and 2 mmol of sodium acetate were added to Schiff base (2 mmol) dissolved in Methanol (10 ml) and subjected to microwave irradiation intermittently at 30 seconds for 4-7 minutes. After completion of the reaction as monitored by TLC, the hot reaction mixture was cooled at room temperature. The solid separated out was filtered, washed thoroughly with methanol and water and dried.
Antimicrobial Activity
Antimicrobial activity of synthesized Schiff bases and their metal complexes were evaluatedin vitro by using agar-plate diffusion technique33 by measuring the zone of inhibition in mm. Theantibacterial activity was evaluated against four bacterial strains Escherichia coli ATCC 25922(E. coli), Pseudomonas aeruginosa ATCC 27853 (P. aeruginosa), Staphylococcus aureus ATCC 25923 (s. aureus) and Bacillus subtilis ATCC 6633 (B. subtilis) at 100μg/mL concentration of samples with standard drugs Streptomycin. After completion of incubation period (18-24 h at 37 0C), the zone of inhibition of growth in the form of diameter in mm was measured (Table 3).
An antifungal activity was evaluated against Aspergillusneiger(A. neiger) and canadidaalbicans(C. albicans) at 100 μg/mL concentration of samples using Fluconazole as standard drug. After completion of the incubation period (18-24 h at 37 0C), the zone of inhibition of growth in the form of diameter in mm was measured (Table 3).
Conclusion
The prepared Schiff bases behave as tridentate chelating agent with Mn(II), Co(II) metal ions and coordinated through N, O and S atoms. The elemental analysis were clearly indicated that ML2 type complexes have 1:2 stoichiometry (M=metal, L=ligand). All the Schiff bases and their Mn(II), Co(II) metal complexes were synthesized by microwave irradiation as well as by conventional method to compare analytical data and confirm their structure. The studies revealed that Schiff bases containing nitro group were found more active against selected bacteria and fungi. It is also observed that Co (II) metal complexes have exhibited higher antimicrobial activity than the respective Schiff bases as cleared from data in table 3.
Acknowledgement
We are thankful to the Principal of Institute and head of the chemistry department for providing necessary facilities to complete this study. One of the authors,Sunita Kumari Bajroliya is also thankful to UGC for financial support in the form of JRF.
References
- Legrand, C.; Ropaa, P.; Vulturescu, B.; Hogeac, A. M.; Surpateanu, G.G.; Cazier, F.; Woisel, P.; Surpateanu, G. Progress in Organic Coatings2005, 53, 106-111
- Ewiss, N. F.; Bahajaj, A. A.; Elsherbini, E. A. J. Heterocycl. Chem.1986, 23, 1451-1458
- Eweiss, N. F.; Bahajaj, A. A. J. Heterocycl. Chem.1987, 24, 1173-1182
- Prasad, A.; Ramalingam, R. J.; Rao, A. B.; Diwan, P. V.; Sattur, P. B. Eur. J. Med.Chem. 1989, 24, 199-201
- Turan-Zitouni, G.; Kaplancıklı, Z. A.; Yıldız, M. T.; Chevallet, P.; Kaya, D. Eur. J. Med. Chem.2005, 40, 607-613
- Chen, J.; Sun, Y.; Chai, K. Y.; Lee, J. S.; Song, M. S.; Quan, Z. S. Bioorg. Med. Chem. 2007, 15, 6775-6781
- Bekircan, O.; Gumrukcuoglu, N. Indian J. Chem. 2005, 44B, 2107-2113
- Turan-Zitouni, G.; Kaplancıklı, Z. A.; Erol, K.; Kilic, F. S. Farmaco1999, 54, 218-223
- Akhtar, T.; Hameed, S.; Al-Masoudi, N. A.; Khan, K. M. Heteroatom. Chem. 2007, 18, 316-322
- Turan-Zitouni, G.; Kaplancikli, Z. A.; Özdemir, A.; Chevallet, P. Arch. Pharm. Chem. Life Sci .2007,340,586-590
- Sarangapani, M.; Reddy, V. M.Indian J. Pharm. Sci.1994,56, 174-177
- Pandeya, S. N.; Sriram, D.; Nath, G.; De Clercq, E. Pharm. ActaHelv.1999, 74, 11-17
- Popp, F. D.; Parson, R.; Donigan, B. E. J. Heterocycl. Chem. 1980, 17, 1329-1330
- Pandeya, S. N.; Sriram D.; DeClercq, E.; Nath, G. Eur. J. Pharm. Sci.1999,9, 25-31
- Singh, G. S.; Singh, T.; Lakhan, R. Indian J. Chem. 1997, 36B, 951-954
- Bhattacharya, S. K.; Chakrabarti, S. Indian J. Exp. Biol. 1998, 36, 118-121
- Islam, M. R.; Abedin, M. J.; Hossain, M. M.; Duddeck H. J. Bangladesh Chem. Soc. 1998,11,71-78
- Kahveci, B. Molecules2005, 10, 376-382
- Kulkarni, D.; Patil, S. A.; Badami, P. S. Journal of Sulfur Chemistry 2009,30(2), 145-159
- Chandak, S.Tetrahedron1995, 51, 10403-10432
- Kidwa, M.; Sapra, P.; Bhushan, K. R.; Saxena, R. K.Montash Chem. 2000, 113, 85-90
- Kappe, C. O.Curr.Opin.Chem. Biol.2002, 6, 314-320
- Larhed, M.; Moberg, C.; Hallberg, A.Acc. Chem. Res. 2002,35, 717-727
- Lidstrom, P.; Tirrey, J.; Wathey, B. Westman J., Tetradedron2001, 57, 9225-9283
- Kalwania, G. S.; Choudhary, S.; Chomal, S. Chemical Science Transactions2014,3(3), 1147-1155
- Mathew, V.; Keshavayya,J.; Vaidya, V. P.J Chem. 2007, 4(3),320-342
- Singh, G.; Singh, P. A.; Singh, K.; Singh, D. P.; Handa, R. N.; Dubey, S. N. Proc. Nat. Acad. Sci. Ind.2002, 72A,87-94
- Hassaan, A. M. A. Indian J. Chem.1997, 36,241-243
- Soliman, A. A.; Linert ,W. Thermochim. Acta1999,333,67-75
- Nakamoto K. Infrared Spectra of Inorganic and Coordination Compounds, Wiley- Interscience.NewYork, (1970)
- Cerchiaro, G.; Saboya, P. L.; Ferreira, A. M. C.; Tomazela, D. M.; Eberlin, M. N. Transit. Met.Chem.2004,29, 495-504
- Murukan, B.; Bhageerethi, S. K.; Kochukittan, M.J. Coord. Chem.2007,60,1607-1617
- Turan-Zitouni, G.; Kaplancikli, Z. A.; Erol,K.; Kilic, F. S.Farmaco1999,54, 218-223

This work is licensed under a Creative Commons Attribution 4.0 International License.









