Synthesis and Characterization of Phosphine and Arsine Complexes of Ruthenium (Ii & Iii) Ligated With 3-(4-Pyridyl)-4-Substituted-Triazoline-5-Thione
R. N. Pandey*1 and Nisha Kumari
P.G. Centre of Chemistry (M.U.), College of Commerce, PATNA – 800020 (India)
DOI : http://dx.doi.org/10.13005/ojc/300480
Article Received on :
Article Accepted on :
Article Published : 01 Jan 2015
Organometallic complexes of ruthenium (II & III) with the formula [RuH(CO)(Ef3)2L] and [RuCl2(Ef3)2L] (E = P/As; L = deprotonated mononegative bidentate 3-(4-pyridyl)-triazoline-5-thione and its 4-phenyl substituted derivative) were synthesized and characterized by elemental analysis, physico-chemical and spectroscopic methods. All new compounds were iso-structural with precursor complexes. Two triphenyl phosphine or triphenylarsine molecules are at trans-disposition and thioamide ligands behaves as bidentate (N, S) donor in assigned octahedral structure.
KEYWORDS:organo-ruthenium (II and III); N; S-chelating thioamide; oh-species
Download this article as:| Copy the following to cite this article: Pandey R. N, Kumari N. Synthesis and Characterization of Phosphine and Arsine Complexes of Ruthenium (Ii & Iii) Ligated With 3-(4-Pyridyl)-4-Substituted-Triazoline-5-Thione. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Pandey R. N, Kumari N. Synthesis and Characterization of Phosphine and Arsine Complexes of Ruthenium (Ii & Iii) Ligated With 3-(4-Pyridyl)-4-Substituted-Triazoline-5-Thione. Available from: http://www.orientjchem.org/?p=6575 |
Introduction
Literature survey reveals that secondary thioamides have versatile coordinating ability and interesting insights into structure and bonding1-3. Bowman-James’s group4-5 and Kanbara and Co-worker6-8 have reported that thioamide based Pincer complexes are photoluminescent and have catalytic activity. The group of Henderson9-11 has reported the synthesis and structural characterization of various transition metal complexes containing chelating thioamide derivatives. The reactions, structures and anti-tumour activity of transition metal ions with thioamide ligands are reported by Mohr et al12. The current interest in organometallic ruthenium complexes with anti-tumour activity13-15 prompted us to investigate this class of complexes with 3-(4-Pyridyl)-4-substituted-Triazoline-5-thione which contains secondary thioamide group. Prior to this work low-valent Pd(0), Pt(0) and Rh(I) complexes of this ligand are reported in our previous communication16. The present study embodies synthesis and characterization of some novel organometallic derivatives of ruthenium (II & III) with 3-(4-Pyridyl)-4-substituted-triazoline-5-thione (I).
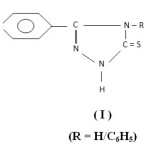 |
Scheme 1 Click here to View Scheme |
Experimental
All chemicals used were either CP grade or AR grade. Solvents were distilled and dried before use. The ligands 3-(4-Pyridyl)-triazoline-5-thione (PytTH) and 3-(4’-Pyridyl)-4-phenyl-triazoline-5-thione (PPytTH) were prepared by the method of Dutta et al17. The ligands were recrystallized from ethanol before use. The precursor complexes, [RuX3(Ef3)3] (X = Cl/Br; E = P/As), [RuH(CO)(Ef3)3Cl] (E = P/As) were prepared following the literature pathway18-22.
Preparation of new ruthenium (II) complexes
All ruthenium (II) complexes were prepared by ligand substitution reactions using precursors [RuH(CO)(Ef3)3Cl] (E = P/As)18-19 and ethanolic solution of ligands, PytTH or PPytTH in equimolar ratio using our previous method23.
Analysis
Sl. No.1 : [RuCl(CO)(PΦ3)2(PytT)] (deep brown) : Calculated (%) for RuC44H35N4OSClP2Ru (865.5) : C = 61.00; H = 5.08; N = 6.47; Ru = 11.66; Found (%) : C = 61.18; H = 5.18; N = 6.50; Ru = 11.72;
Sl. No.2 : [RuCl(CO)(AsΦ3)2(PytT)] (Brown) : Calculated (%) for RuC44H35N4OSAs2Cl (953.34) : C = 55.38; H = 3.67; N = 5.87; Ru = 10.59; Found (%) : C = 55.42; H = 3.77; N = 5.92; Ru = 10.62;
Sl. No.3 : [RuCl(CO)(PΦ3)2(PPytT)] (yellowish brown) : Calculated (%) for RuC50H39N4SP2Cl (941.50) : C = 63.72; H = 4.14; N = 5.95; Ru = 10.72; Found (%) : C = 63.82; H = 4.21; N = 6.02; Ru = 10.77;
Sl. No.4 : [RuCl(CO)(AsΦ3)2(PPytT)] (yellowish brown) : Calculated (%) for RuC50H39N4SAs2Cl (1025.5) : C = 58.50; H = 3.80; N = 5.46; Ru = 9.84; Found (%) : C = 58.62; H = 3.91; N = 5.38; Ru = 9.92;
Preparation of ruthenium (III) complexes
All the reactions were carried out strictly anhydrous conditions and the complexes were prepared using a general method. Benzene solution of [RuX3(Ef3)3] (X = Cl/Br; E = P/As) and ligand were mixed in equimolar ratios and stirred on magnetic stirrer for 15 mints and heated on reflux till the brown colour changed to yellowish green. The working mixture was concentrated and cooled. Addition of light petroleum ether produced solid products which was filtered and dried in vacuo over anhydrous CaCl2. (yield = 76%)
Analysis
Sl. No.5 : [RuCl2(PΦ3)2(PytT)] (yellowish green) : Calculated (%) for RuC43H35N4P2SCl2 (873) : C = 59.10; H = 4.00; N = 6.41; Ru = 11.56; Found (%) : C = 59.01; H = 3.98; N = 6.38; Ru = 11.49;
Sl. No.6 : [RuCl2(AsΦ3)2(PytT)] (greenish yellow) : Calculated (%) for RuC43H35N4As2SCl2 (960.84) : C = 53.70; H = 3.64; N = 5.82; Ru = 10.51; Found (%) : C = 54.01; H = 3.70; N = 5.78; Ru = 10.60;
Sl. No.7 : [RuBr2(PΦ3)2(PytT)] (brownish green) : Calculated (%) for RuC43H35N4P2SBr2 (962) : C = 53.63; H = 3.63; N = 5.82; Ru = 10.49; Found (%) : C = 55.63; H = 3.68; N = 5.80; Ru = 10.52;
Sl. No.8 : [RuBr2(Asf3)2(PytT)] (brownish green) : Calculated (%) for RuC43H35N4As2SBr2 (1049.74) : C = 49.15; H = 3.33; N = 5.33; Ru = 9.62; Found (%) : C = 49.32; H = 3.40; N = 5.23; Ru = 9.68;
Sl. No.9 : [RuCl2(Pf3)2(PPytT)] (yellowish green) : Calculated (%) for RuC49H39N4SP2Cl2 (949) : C = 61.95; H = 4.10; N = 5.90; Ru = 10.64; Found (%) : C = 62.11; H = 4.20; N = 6.01; Ru = 10.68;
Sl. No.10 : [RuCl2(AsΦ3)2(PPytT)] (yellowish green) : Calculated (%) for RuC49H39N4SAs2Cl2 (1033) : C = 56.92; H = 4.74; N = 6.42; Ru = 9.77; Found (%) : C = 57.01; H = 4.80; N = 5.50; Ru = 9.89;
Sl. No.11 : [RuBr2(PΦ3)2(PPytT)] (greenish brown) : Calculated (%) for RuC49H39N4SP2Br2 (1037.8) : C = 56.65; H = 3.75; N = 5.39; Ru = 9.73; Found (%) : C = 56.66; H = 3.88; N = 5.42; Ru = 9.80;
Sl. No.12 : [RuBr2(AsΦ3)2(PPytT)] (greenish brown) : Calculated (%) for RuC49H39N4SAs2Br2 (1125.64) : C = 52.23; H = 3.46; N = 4.97; Ru = 8.97; Found (%) : C = 52.40; H = 3.50; N = 5.01; Ru = 9.00;
The analysis of C, H and N were performed at CDRI, Lucknow, India. IR spectra of ligand and complexes were recorded on a Perkin Elmer 577 spectrophotometer in the range of 4000-200 cm-1 as KBr pellet technique. Electronic spectra of ligand and complexes were recorded on a Backmann Du-6 spectrophotometer. The molar conductance of complexes (10-3 M) were measured in DMF using Wiss-Werkstatter Weihem obb type LBR conductivity meter. The magnetic susceptibility was measured on a gouy balance using Hg[CO(SCN)4] as calibrant.
Results And Discussion
The reactions of [RuHCl(CO)(Ef3)2] (E = P/As) (Scheme I) and [RuX3(Ef3)3] (X = Cl/Br; E = P/As) with thioamide ligands (Scheme II) in 1:1 molar ratio in benzene afforded new hexa-coordinated complexes and ligands acts as mononegative bidentate anion.
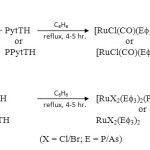 |
Scheme 1,2 Click here to View scheme |
The analytical data are in good agreement with general molecular formula proposed. The mononegative bidentate thioamide ligands replaces are triphenyl phosphine or triphenyl arsine molecule, one chlorido or bromido group from precursors [RuX3(EΦ3)3] and hydrido group and one PΦ3 or AsΦ3 from precursors, [RuHCl(CO)(EΦ3)3]. All the isolated products were air-stable and non-hygroscopic nature and fair soluble in acetone, EMK, DMF & DMSO.
Magnetic moment and UV-Vis Spectra
The substitution products [RuCl(CO)(EΦ3)2(PytT)] and [RuCl(CO)(Ef3)2(PPytT)] were diamagnetic indicating Ru++ (d6) and 1A1g ground state. The d – d transition bands (table 2) consistent with octahedral structure24.
The magnetic moment value of [RuX2(EΦ3)2(PytT)] or [RuX2(Ef3)2(PPytT)] (X = Cl/Br; E = P/As) are in the range of 1.80-1.95 BM corresponding to a single unpaired electron in a low spin T2g5eg0 configuration and oxidation state of ruthenium is +3. So, the ground state of Ru (III) is 2T2g and first excited doublet level in order of increasing energy are 2A2g and 2A1g which arises from t2g4eg1 configuration25. The electronic spectra of complexes exhibit bands around 18585 (2T2g →2A2g), 22935 (T2g → π*, MLCT), 38760 (n→ π*) and 49020 cm-1 (p →π*) are consistent with octahedral structure of other ruthenium (III) complexes26.
Infrared Spectra
The IR bands of interest of ligands and complexes are given in Table 1. A comparison of the infrared bands of the ligands and complexes indicate the simultaneous formation of Ru – N and Ru – S bonds.
- Two bands having contribution from nNH and nCH are present at 3120 cm-1 and 3080 cm-1 in PytTH and at 3060 cm-1 in PPytTH. But there is only one band centred at 3080 cm-1 in the spectra of complexes of PytTH and found to be absent in the complexes PPytTH. This suggests that H-atom of N-H group of ligands has been replaced by the Ru(II) and Ru(III) ions leading to the formation of RuII-N and RuIII-N bonds.
- Thioamide band IV has main contribution from vC …– S observed at 850 cm-1 (PytTH) and at 785 cm-1 (PPytTH) in the spectra of the ligands shift to lower frequency by 30-50 cm-1 indicating coordination of thioamide ligands through thione sulphur and formation of ruthenium – S bond following our previous observations26.
- The simultaneous Ru-N and Ru-S bonding results in a decrease in the frequency of thioamide band IV by 30-50 cm-1, blue shift to higher frequency of 10-15 cm-1 of band II and red shift to the extent of 35-55 cm-1 in thioamide band I. These observations are in good agreement with previous literature27. New bands at 435-465 cm-1 (Ru-N) and 405-410 cm-1 (Ru-S) due to stretching modes further supports these observations.
- The presence of bands near 540, 680, 750 and 1550 cm-1 are attributed to coordinated PΦ3/AsΦ3 in complexes29-30. The presence of single Ru-P stretching modes at 510-500 cm-1 confirms two bulkyu PΦ3 groups are at mutual Trans-disposition in octahedral structure of complexes (Str. I & II). However, more than one Ru-X stretching mode in Ru (III) complexes indicates cis-disposition of two chlorine or two bromine atoms in octahedral structure (Str. III & IV).
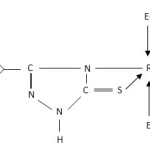 |
Figure1: (X = Cl/Br; E = P/As) |
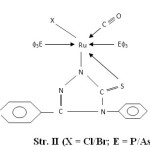 |
Figure2: (X = Cl/Br; E = P/As) |
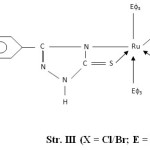 |
Figure3: (X = Cl/Br; E = P/As) |
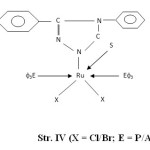 |
Figure4: (X = Cl/Br; E = P/As) |
5. The non-ligand band at 1950-1960 cm-1 in carbonyl complexes of ruthenium (II) supports terminal C º O group slightly higher frequency than in precursor complex.
Thus, the ligands behaves as mononegative bidentate (N, S) donor.
Table-1 : Assignment of Major infrared bands of 3-(4-Pyridyl)-4-substituted-triiazoline-5-thioneand their Ru (II & III) complexes in (cm-1).
|
Compounds |
nN-H + nCH |
Thioamide Bands |
nRu-S |
nRu-N |
nRu-X |
|||
|
Band I |
Band II |
Band III |
Band IV |
|||||
|
PytTH (ligand) |
3120 m 3080 m |
1570 (sb) |
1205 (s) |
960 (s) |
850 (ms) |
¾ |
¾ |
¾ |
|
[RuIICl(CO)(PΦ3)2(PytT)] |
3080 m |
1535 (s) |
1210 (s) 1200 (m) |
925 (m) |
810 (m) |
406 w |
441 (m) |
370 w 340 w |
|
[RuIICl(CO)(AsΦ3)2(PytT)] |
3085 m |
1530 (s) |
1220 m 1200w |
935 (m) |
820 (m) |
410 w |
445 (m) |
370 w 350 w |
|
[RuIIICl2(PΦ3)2(PytT)] |
3060 m |
1545 (s) |
1215 m 1200 m |
945 (m) |
830 (m) |
425 (m) |
448 (m) |
364 m 343 m |
|
[RuIIICl2(AsΦ3)2(PytT)] |
3070 m |
1540 (s) |
1215 (m) 1200 (m) |
940 (m) |
825 (m) |
430 m |
450 m |
356 m 335 m |
|
[RuIIIBr2(PΦ3)2(PytT)] |
3065 m |
1535 (s) |
1200 w 1185 m |
945 (m) |
820 (m) |
430 w |
445 m |
355 m 330 m |
|
[RuIIIBr2(AsΦ3)2(PytT)] |
3070 m |
1545 (s) |
1220 m 1190 m |
950 (s) |
825 (m) |
430 w |
430 m |
360 m 330 m |
|
PPytTH (ligand) |
3060 m |
1480 (s) |
1290 (s) 1240 (m) |
1090 (m) |
785 m |
¾ |
¾ |
¾ |
|
[RuIICl(CO)(PΦ3)2(PPytT)] |
1435 (s) |
1235 (s) 1210 (m) |
1035 (m) |
765 m |
428 w |
464 |
364 m |
|
|
[RuIICl(CO)(AsΦ3)2(PPytT)] |
1460 (s) |
1240 (s) 1220 (m) |
1040 w |
765 m |
436 (w) |
458 m |
356 |
|
|
[RuIIICl2(PΦ3)2(PPytT)] |
1445 m |
1250 (s) 1230 (m) |
1065 (m) |
770 (m) |
440 w |
476 |
365 m 345 |
|
|
[RuIIICl2(AsΦ3)2(PPytT)] |
1440 (ms) |
1245 (s) 1235 (m) |
1070 (m) |
772 (m) |
436 w |
458 m |
356 m 335 m |
|
|
[RuIIIBr2(PΦ3)2(PPytT)] |
1465 (m) |
1250 m 1235 m |
1065 (m) |
760 m |
440 w |
460 m |
350 m 330 m |
|
|
[RuIIIBr2(AsΦ3)2(PPytT)] |
1460 (m) |
1250 m 1220 (m) |
1065 (m) |
765 m |
438 w |
470 m |
345 m 332 m |
|
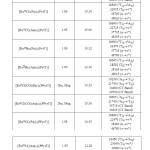 |
Table2 : Magnetic, Conductometric and Electronic spectral data of ruthenium (II & III) complexes Click here to View table |
References
- Jagodzinski, T.S., Chem, Rev. 103, 197 (2003).
- Pandiarajan, D. and Ramesh, R., J. Organometallic Chem. 723, 26 (2013).
- Murai, T., Top. Curr. Chem. 251, 247 (2005).
- Begum, R.A., Powell, D., Bowman-James, K., Inorg. Chem. 45, 964 (2006).
- Hossain, M.A., Lucarini, S., Powell, D. & Bowman-James, K., Inorg. Chem. 43, 7275 (2004).
- Kanbara, T., Okada, K., Yamamoto, T., Ogawa, H. and Inoue, T., J. Organomet. Chem. 689, 1860 (2004).
- Okamoto, K., Kanbara, T., Yamamoto, T. and Wada, T., Organometallics, 25, 4026 (2006).
- Koizumi, T., Teratani, T., Okamoto, K., Yamamoto, T., Shimoi, Y. and Kanbara, T., Inorg. Chem. Acta, 363, 2474 (2010).
- Henderson, W., Nicholson, B.K. and Tiekink, E.R.T., Inorg. Chim. Acta, 359, 204 (2006).
- Henderson, W. and Rickard, C.E.F., Inorg. Chim. Acta, 343, 74 (2003).
- Henderson, W., Nicholson, B.K., Dinger, M.B. and Bennett, R.L., Inorg. Chim. Acta, 338, 210 (2002).
- Alagoz, C., Brauer, David J. and Mohr Fabian, J. Organometallic Chem. 694, 1283 (2009).
- Clarke, M.J., Coord. Chem. Rev. 236, 209 (2003).
- Gielen, M., Tiekink, E.R.T., Metallotherapeutic Drugs and Metal-based Diagnostic Agents, John Wiley and Sons, Chichester, 2005.
- Yan, Y.K., Melchart, M., Habtemariam, A. and Sadler, P.J., Chem. Commun. 4764 (2005).
- Pandey, R.N., Kumar Arun, Singh, R.S.P., Sahay, A.N. and Kumar, Shashikant, J. Indian Chem. Soc. Vol 69, 804 (1992).
- Dutta, S., Acharya, A.K. and Basu, U.P., J. Indian Chem. Soc., 45, 338 (1968).
- Ahmad, N., Levison, J.J., Robinson, S.D. and Uttley, M.F., Inorg. Synth. 15, 45 (1975).
- Balasubramanian, K.P., Manivannanand, S. and Chinusamy, V., J. Ultra Chem. 4, 15 (2008).
- Poddar, R.K., Khullar, I.P. and Agarwala, U., J. Inorg. Nucl. Chem. Lett. 10, 221 (1974).
- Natarajan, K., Poddar, R.K. and Agarwala, U., J. Inorg. Nucl. Chem. 39, 431 (1977).
- Chatt , J., Leigh, C.J., Mingos, DMP and Paske, R.J., J. Chem. Soc. A, 2636 (1968).
- Pandey, R.N., Bala, Renu and Sinha, A.K., Oriental J. Chem. Vol 27(1), 293 (2011).
- Pandey, R.N. and Gautam, K.V., J. Ultra Chem. Vol 10(1), 35 (2014).
- Pandiarajan, D. and Ramesh, R., Polyhedron, 34, 136 (2012).
- Kannan, S. and Ramesh, R., Polyhedron, 25, 3095 (2006).
- Singh, B., Singh, R., Choudhary, R.V. and Thakur, K.P., Indian J. Chem. 11, 174 (1973).
- Singh, B. and Singh, R., J. Inorg. Nucl. Chem. Vol 34, 3449 (1972).
- Pandiarajan, D. and Ramesh, R., J. Organomet. Chem. 723, 26 (2003).
- Kumar, K.N., Ramesh, R. and Liu, Y., J. Mol. Catal. A Chem., 265, 218 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









