Study of Stability Constants of Fe (Iii) And Mn (Ii) With Chloramphenicol by Paper Electrophoretic Technique
Arvind Singh and O. P. Rai*
Department of Chemistry, Govt. (Autonomous) P. G. College, Satna 485001 (M.P.), India
DOI : http://dx.doi.org/10.13005/ojc/300472
Stabilty constant of binary complexes of Fe(III) and Mn(II) with medicinally important ligand chloramphenicol antibiotics in solution were determined by paper electrophoretic technique. Stability constant of the complexes were determined at 25°C temperature and 0.1M (HClO4) ionic strength. Our study is based upon the migration of a spot of metal ions on a paper strip at different pH against mobility gives information about the binary complexes and permits to calculate their stability constant. The stability constant data revealed that chloramphenicol may be used as chelating agent in chelation for medical treatment of metal overload or poisoning.
KEYWORDS:Paper electrophoresis; Binary complexes, Fe(III), Mn(II); Chloramphenicol, Chelation; Stability constants
Download this article as:| Copy the following to cite this article: Singh A, Rai O. P. Study of Stability Constants of Fe (Iii) And Mn (Ii) With Chloramphenicol by Paper Electrophoretic Technique. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Singh A, Rai O. P. Study of Stability Constants of Fe (Iii) And Mn (Ii) With Chloramphenicol by Paper Electrophoretic Technique. Available from: http://www.orientjchem.org/?p=5676 |
Introduction
Metals are an integral part of many structural and functional component of human body and play an important role in biological systems. Metal ions are required for many critical functions, physiological and pathological processes in human1-2. Metals have been used in the treatment of many diseases of humans since ancient times3. The effective coordination of metal ions with ligands may have significance in biological implication4. The chelation therapy for intoxication of metals depends upon the chelating agent being able to reach the intercellular site where the metals are firmly bound. For a pretty long time, varieties of ligand have been used as chelating agent to combat metal poisoning. Here Chloramphenicol [2, 2-dichloro-N-{(1R, 2R)-2-hydroxy-1-(hydroxymethyl)-2-(4 nitrophenyl)ethyl}-acetamide] a bacteriostatic-antimicrobial with formula C11H12Cl2N2O5 having molecular mass 323.1320g/mol has been used as ligand. The chemistry of Metal-drug coordination compounds is more popular now than before particularly in the design of more biologically active drugs5. Metal ions are known to affect the action of many drugs. Many drugs posses modified pharmacological and toxicological properties when administered in the form of metallic complexes. When dealing with the interaction between drugs and metal ion present in living system. The efficacies of the drug on coordination with a metal are enhanced in many cases6.
Iron and manganese are essential for normal biological and physiological functions in human beings. The amount of these metals can be varying in human body through intake of diet and different environmental factors. They may either contribute towards the health of the human body or cause toxicity7.
Literature survey8-12 reveals that no work has been done on chelating tendencies of chloramphenicol with transition metal ion Fe(III) and Mn(II) in solution. This paper describes a paper electrophoretic method for the determination of stability constant of these complexes in solution. Stability constant is useful physical entity, which explains the importance and function of various complexes in biological systems. Thus useful information may be obtained from stability data relating to metal ions and ligand. A significant development on the determination of stability constants of complexes was made by Jokl13 in 1964. A theoretical treatment similar to that of Jokl was adopted by Biernet14 for the stepwise complex formation.
Experimental
Instrument
A Systronic (Naroda, India) Model 610 electrophoresis system was used. The apparatus consisted of a PVC molded double tank vessel. It has a built-in power supply (a.c.–d.c.) that is fed directly to a paper electrophoresis tank. In our laboratory significant change in the instrument has been made. Two hollow rectangular metallic plates coated with thin polyethylene sheets on the outer surface have been used through which thermo stated water is run for controlling the temperature (25°C). The tanks were closed with a transparent PVC molded lid. The whole assembly is tight which prevent moisture changes, which may upset the equilibria in a paper strip. The assembly design keeps to a minimum the disturbing effects of evaporation from the unwanted liquid flow in the paper. Each electrolyte tank contains a separate electrode chamber in which the anode and cathode are placed, respectively. The pH measurements were made with an Elico (Hyderabad, India) Model L1–10 pH meter using a glass and calomel electrodes assembly, working on 220 V/50 Hz established a.c. mains.
Chemicals
Metal perchlorate were prepared by precipitation of metal carbonates from 0.1 M solution of Chlorides of Fe(III) and Mn(II) (A.R. grade) with the solution of sodium carbonate. The precipitate were thoroughly washed with boiling water and treated with a calculated amount of 1% perchloric acid. These were boiled on a water bath and filtered. The metal contents of the filtrate were determined and final concentration kept at 5.0 × 10-3 M. The solution were standardized and diluted with distilled water as required.
Filter Paper Strips
Strips of Whatman No. 1 filter paper for chromatography (30×1) cm² were used and the potential gradient was 6.6 V/cm.
Electro–Osmotic Indicator
5.0 × 10-3 M glucose (BDH A. R. grade) was prepared in water and used as an electro–osmotic indicator for the correction due to electro–osmosis.
Detecting Reagent for Metal ions
Ammonium thiocyanate for Fe(III) and 0.1% (W/V) solution of 1–(2-pyridylazo)–2-naphthol (PAN) in ethanol for Mn(II) was used.
Indicator for Glucose
A saturated aqueous solution (0.9 ml) of silver nitrate was diluted with acetone to 20 ml. Glucose was detected by spraying with this solution, and then with 2% ethanolic hydroxide when a black spot was formed.
Background Electrolyte
Stock solution of (5.0 M) was prepared by suitable dilution of 70% perchloric acid (SDS A. R. grade). 2.0 M sodium hydroxide (A. R. grade) and 0.5 M stock solutions of the complexing reagent Chloramphenicol (A. R. grade) solutions were prepared. All chemicals were used without further purifications. Each solution was standardized using the appropriate method. The background electrolyte used in the study of binary complexes was 0.1M perchloric acid and 0.01M chloramphenicol. The system was maintained at various pH by the addition of sodium hydroxide.
Procedure
The midpoint of each Whatman filter paper strip was marked for recording the mobility observation of a particular metal ion. Each of the two electrolyte vessels was filled with 150 ml of background electrolyte containing 0.1 M perchloric acid and liganding reagent (1.0×10-2 M). The paper becomes moistened with the background electrolyte solution due to diffusion. Then the spot of each metal solution was applied at the marked mid-point of the strips using micropipette. At least one strip was spotted with glucose solution for electro-osmotic correction. The second insulated plate was placed on the paper strips and then thermostated water (25oC) was circulated in the plates to keep the temperature constant. The lid was, then, placed on the instrument to make it air tight. It was left for 10 minutes to insure wetting of paper strips. Subsequently, a direct 200 volts was applied between the electrodes. Eletrophoresis was carried out for 30 minutes after which these strips were removed from the tank and dried. The metal ions and glucose spots were detected by specific reagents. The leading and tailing edges were measured from the mid-point and the mean were taken. The distance moved by glucose spot was subtracted (in case of migration towards anode) to obtain the correct path length. Migration towards anode and cathode were designated by negative and positive signs, respectively.
Electrophoretic observations on metal ion spots were recorded at various pH values of the background electrolyte obtained by adding sodium hydroxide solution, the ionic strength being maintained at 0.1M. The observed mobility of the migrant was calculated by using the formula.
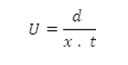
After applying the correction factor, the observed mobility is given as
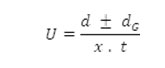
Where U = mobility of metal ion/complex ion, d = mean of duplicate distances travelled by metal ion/complex ion, dG = mean of duplicate distances travelled by glucose spots, x = field strength and t = time for electrophoresis.
The electrophoresis was carried out for 30 minutes as for metal ions. The mobility of the metal ion spots on the strips were reported along with pH values. The individual mobility of the duplicate spots was fairly equal and the variation was always less than 0.5 cm.
Result and Discussion
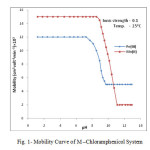 |
Fig1: Mobility Curve of M –Chloramphenicol System Click here to View figure |
The electrophoretic mobility of metal ion spot against pH gives number of plateaus shown in figure 1. A plateau is obviously show pH range where mobility is practically constant. In the region of first plateau metal ions are uncomplexed. It lies in low pH region where concerned of highly protonated species of ligand is obviously maximum, hence it is concluded that this protonated species of ligand is not complexing. Beyond this pH range metal ion spot has progressively decreasing mobility. This decrease shows formation of complex of metal ion with ligand. A point is reached beyond which mobility of the metal ion species remain constant. This is the second plateau which corresponds to a pH region in which 1:1 cationic complex is formed. One ligand anion combines with each metal ion to form [Fe(L)]++ and [Mn(L)]+, cationic complexes with Fe(III) and Mn(II) respectively. On further increase of pH beyond the second plateau there is no further decrease in mobility of metal ion indicates no further complexation takes place. It is significant that these studies give clear evidence of the binary complex formation of 1:1 composition. The complexation of metal ions with ligand anion [L–] can be represented as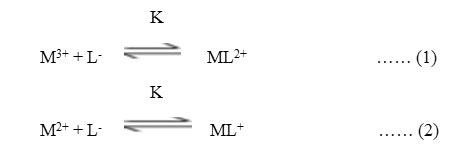
Where M3+ = Fe3+ and M2+ = Mn2+ metal ion,
[L–] = chloramphenicol anion,
K = stability constants
The spot is moving under the influence of electric field, if non-protonated and protonated species are considered, the overall mobility can be given by expression as
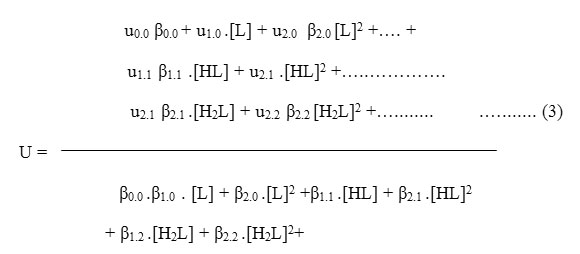
Where u0.0 is the speed of uncomplexed metal ion, u1.0 is that of the complex formed by the combination of one unprotonated anionic ligand with the metal ion and ux.p is the speed of the metal complex formed by the combination of x anions containing p protons each. β’s are the overall stability constants of the different metal complexes formed in the interaction. On taking into consideration different equlibria above equation transformed into following useful form:

Where, u0 and u1 are the ionic mobilities of uncomplexed metal ions and 1:1 metal complexes respectively. For calculating stability constant K, the region between first and second plateau is pertinent. The system contains using the principle of average mobility. Stability constant can be calculated with the help of mobility of first plateau and second plateau the system contains overwhelmingly a mixture of free metal ion and 1:1 complex. Using the principle of average mobility
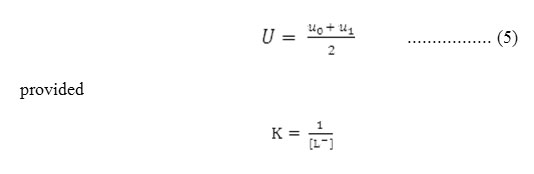
Accordingly the pH corresponding to the average value of u0 and u1 is found from the figures and with the knowledge of dissociation constant of ligands, the concentration of ionic ligands at this pH is calculated. Its reciprocal gives the stability constant K of the 1:1 complex. The concentration of chelating ligands anion [L–] is determined as:

Where [Lt] = total concentration of the ligand chloramphenicol
k = dissociation constant of chloramphenicol
With the help of dissociation constant of chloramphenicol (pka =11.03). The concentration of chloramphenicol anion is determined for the pH, from the which stability constant (K) is calculated. The calculated stability constants value for 1:1 binary complexes are given in table 1 which follows the Irving William’s15 order for stability constants of transition metals of the first transition series and no comparison can be made with the literature value due to its unavailability.
Table – 1: Stability Constant of M – Chloramphenicol Binary Complexes
| Metal ions |
Stability Constants |
|
|
Calculated Values Log K |
Literature values Log K |
|
|
Fe (III) |
4.1331 |
– |
|
Mn (II) |
2.9782 |
– |
Conclusion
Paper electrophoretic technique is very useful technique for determination of stability constant of metal complexes in solution. Fe(III) and Mn(II) are present in many structural and functional part in the human body and essential for normal physiological processes, but they are toxic if present in excess. Chloramphenicol may be used to reduce the levels of these metal ions in living cells. Chelation and stability constant are important parameter for formation of metal complexes.
References
- Alimarin, I. P. and Sheleskaya, V. I., Pure Appl. Chem., 21, 461 (1970).
- Janes, D. L. and Margerum,W. M., Inorg. Chem., 5, 1135 (1966).
- Orvig, C. and Abrams, M. J., Medicinal Inorganic Chemistry, Chemical Reviews, 99, 2201-2204 (1970).
- Prakash, O.; Krishan,B.; Jacob,G.; Orient.J.Chem. 2013, 29(2), 823-828.
- A.O. Ajibola, Essential Medicinal Chemistry, 2nd Edn., Shaneson Jeresey, 2b (1999).
- Diaj, G. M., Alanso, R. P. and Esparza, R. M., J. Inorg. Biochem., 64, 207 (1996).
- Obaleya, J. A., Nde-aga, J. B. and Balogun, E.A., Afr. J. Sci., 1997, 1, 10-12 .
- Yambe, S. and Koho, J. P., Chem. Abs., 1989, 111, 219307z .
- Tewari, B. B., Journal of Chromatography A, 2002, 962, 233-237 .
- Tewari, B. B., J. Mex. Chem. Soc., 2008, 52(3), 219-223 .
- Tewari B. B., Revista Bolivina De Quimica., 2008, 25(1), 4-9 .
- Sachan, S. et al, Croat. Chem. Acta, 2011, 84(4), 461-464 .
- Singh, P. P. and Kanaujia, S., Chem. Sci. Trans., 2013, 2(3), 1028-1034 .
- Jokl, V. J., Chromatography, 1964, 14, 74.
- Biernet, J., Rocz. Chem., 1964, 38, 343 .
- Irving, H. and Williams, R. J. P., Nature, 1948, 162, 746 .

This work is licensed under a Creative Commons Attribution 4.0 International License.









