Studies on the Cu (II) and Co (II) complexes with N,N’–bis (2-benzothiazolyl) -2,6 pyridine dithiocarboxamides.
A. K. Srivastava*, Swati Kumari, and Nidhi Srivastava
Department of Chemistry, B. R. A. Bihar University, Muzaffarpur – 842001, Bihar, India
DOI : http://dx.doi.org/10.13005/ojc/300473
Article Received on :
Article Accepted on :
Article Published : 14 Dec 2014
The ligand N,N’–bis (2-benzothiazolyl)-2,6–pyridine dithiocarboxamides and its Cu (II) and Co (II) complexes were synthesized. Their structures were elucidated on the basis of elemental analysis, analytical data, magnetic moments, electronic spectral and IR Spectral studies. The complexes were found to be monomeric and nonelectrolytic in nature. On the basis of electronic spectral studies the complexes were concluded to be distorted octahedral. The IR Spectrum suggested that the ligand behaved as uninegative bidentate ligand coordinating through nitrogen and sulphur atoms.
KEYWORDS:N; N’–bis(2-benzothiazolyl)-2;6–pyridine dithiocarboxamide; 2; 6–pyridine dithiocarboxylic acid; 2–aminobenzothiazole; spectral study;
Download this article as:| Copy the following to cite this article: Srivastava A. K, Kumari S, Srivastava N. Studies on the Cu (II) and Co (II) complexes with N,N’–bis (2-benzothiazolyl) -2,6 pyridine dithiocarboxamides. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Srivastava A. K, Kumari S, Srivastava N. Studies on the Cu (II) and Co (II) complexes with N,N’–bis (2-benzothiazolyl) -2,6 pyridine dithiocarboxamides. Available from: http://www.orientjchem.org/?p=5834 |
Introduction
The coordination chemistry of amide ligand is an important part of a number of chemical investigations. Sigel and Martin1 reviewed the structure and stability of metal ion complexes of amide, oligopeptides and related ligands. In recent years, pyridine carboxamides, a burgeoning class of multidentate ligands have received much importance because these ligands have found use in asymmetric catalysis,2,3 molecular receptors,4,5 dendrimer synthesis6. Many derivatives of pyridine dithiocarboxamides show anti inflammatory, antipyretic and analgesic activities7-8.
In this paper the synthesis of N,N’–bis (2-benzothiazolyl) -2,6–pyridine dithiocarboxamide(I) and isolation of their Cu(II) and Co(II) complexes are reported. The complexes were characterized using elemental analysis, magnetic moments, UV – Visible spectra and IR Spectral Studies.
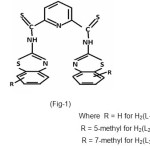 |
Figure 1 Click here to View figure |
Material and Methods
The solubility of all complexes were tested in cold and hot water, in common organic solvents and all the complexes were found to be insoluble.
UV – Vis spectrum was recorded in solid state by diffuse reflectance method due to insoluble nature of complexes using Barium sulphate as inert material. IR spectra were obtained on Perkin Elmer 157 or 577 model in nujol mull or Potassium bromide/ Cesium iodide disc. The estimation of Cu and Co were carried out in our laboratory by using Vogel’s method9.
Synthesis of Ligand BPD
2,6 – Pyridine dithiocarboxylic acid (1.99 g, 10mmol) and thionyl chloride (20-25 ml) were refluxed under anhydrous conditions for 4-6 h. The excess thionyl chloride was removed under reduced pressure and the remaining mixture was cooled at 00C and dry pyridine (30-35 ml) was added followed by 2-aminobenzothiazole (3.0 g, 20 mmol) with occasional stirring, until the evolution of HCl had ceased. The solid obtained was poured into ice cold water (200 ml), filtered off and washed with 5% NaHCO3 solution, then with hot water and ethanol. The resulted black crude product was recrystallized from dioxane. The purity of compound was checked by TLC.
Synthesis of the Complexes
A solution of 5mmol of MCl2 [where M = Cu (II) and Co (II) ] in ethanol (10ml) was added to the ligand (2.31g, 5mmol) suspended in nitrobenzene (10ml) and the mixture was refluxed for 12h. The obtained precipitate was filtered off, washed with water and ethanol and dried in air. All the isolated complexes were insoluble in most organic solvents, but soluble in DMF and DMSO.
The complexes decompose without melting when heated above 3100C.
Results and Discussion
Elemental analysis showed that formula of ligands was C21H13N5S4(H2L1), C23H17N5S4(H2L2) and the formula of complexes were ML2 where M = Cu (II) and Co (II) ions. The complexes were insoluble in water, benzene and other common solvents but were soluble in DMSO and DMF. The complexes were characterized by IR and UV absorption data.
IR Spectra
The infrared spectrum of the ligand showed a band around 3254 cm-1 which was assigned to vNH vibration. The N-H stretching appeared in all complexes as a very weak band. While ˃ C = S group of ligand absorbed around 1180 cm-1 and exhibited medium strong bands and ˃C = S group of complexes registered a lower shift of around 25 to 27 cm-1 and appeared at 1155cm-1 as medium band. This indicated thio enolisation of ˃ C = S group during the complex formation.
The benzothiazole absorption and pyridine ring absorption of ligand is also merged with the thio group and therefore a strong and medium absorption is displayed. The pyridine ring stretching of complexes registered a lower shift (except the first band) which indicated the involvement of N -atom in the ring coordination.
Electronic Spectra
The electronic spectral bands in ligands were obtained at around 273 nm and 359 nm which might have arose due to n —> π* and π —> π* transitions.
The electronic spectral bands in Cu (II) observed around 360 nm appeared due to 2B1g —> 2B2g mixed with internal ligand transition and the 2B1g —>2Eg transition coupled with internal ligand transition and charge transfer transition occurred at 275 nm. The only d-d transition of metal ion observed around 724 nm appeared to arise due to 2B1g —> 2A1g transition.
In Co (II) complexes the electronic spectral bands (excluding the internal ligand transitions) were identified at around 512 nm and 460 nm and they were supposed to arise due to 4T1g (F) —> 4A 2g and 4T1g (F) —> 4T 1g (P) transitions of approximately high spin octahedral Co (II) geometry. The Covalent character and 10Dq were calculated on the basis of data10 and found as
β = 0.95, β0 = 5%, 10Dq = 13109cm-1
Table – 1 : Physical Constant and Elemental Analysis of Compound
| Compound | Colour | M.P. | Yield |
Elemental analysis |
|||||||
|
M |
N |
S |
C |
||||||||
|
Found |
Cal |
Found |
Calc. |
Found |
Calc. |
Found |
Calc. |
||||
| H2 (L1) | Black | 2890C | 90% |
– |
– |
15.11 |
15.10 |
27.64 |
27.84 |
54.42 |
54.52 |
| H2 (L2) | Deep Brown | 2940C | 89% |
– |
– |
14.25 |
14.28 |
26.06 |
26.09 |
56.21 |
56.28 |
| H2 (L3) | Light Brown | 2980C | 88% |
– |
– |
14.26 |
14.27 |
26.07 |
26.08 |
56.20 |
56.23 |
| Cu (L1)2 | Deep BrownBlack | ˃3000C | 78% |
6.41 |
6.43 |
14.15 |
14.17 |
25.90 |
25.92 |
51.03 |
51.06 |
| Cu (L2)2 | Dark Brown | ˃2950C | 76% |
6.11 |
6.08 |
13.44 |
13.42 |
24.56 |
24.54 |
52.90 |
52.92 |
| Cu (L3)2 | Dark Brown | 3100C | 75% |
6.10 |
6.08 |
13.40 |
13.42 |
24.51 |
24.54 |
52.95 |
52.92 |
| Co (L1)2 | Light Pink | 3050C | 80% |
6.02 |
6.00 |
14.26 |
14.24 |
26.06 |
26.04 |
51.29 |
51.27 |
| Co (L2)2 | Dark Pink | 3000C | 85% |
5.68 |
5.67 |
13.48 |
13.47 |
24.66 |
24.63 |
53.15 |
53.12 |
| Co (L3)2 | Dark Pink | 2950C | 85% |
5.69 |
5.67 |
13.45 |
13.47 |
24.65 |
24.63 |
53.14 |
53.12 |
Table – 2: Characteristics iR Band (in Cm-1) of Ligand and Complexes
| Ligand | N-H | ˃C=S | Thiocarboxamide |
Benzothiazole |
Pyridine ring stretching |
|||
|
|
|
|||||||
|
I |
II |
III |
IV |
|||||
| H2 (L1) | 3252(m) | 1178(s) | 1396(m),1049(m),786(w) |
1610(s) |
1609(m) |
1540(m) |
1457(m) |
1444(m) |
| H2 (L2) | 3256(m) | 1180(s) | 1398(m),1052(m),789(w) |
1612(s) |
1615(m) |
1545(m) |
1460(m) |
1449(m) |
| H2 (L3) | 3253(m) | 1182(s) | 1402(m),1054(m),793(w) |
1614(s) |
1618(m) |
1549(m) |
1464(m) |
1453(m) |
| Cu (L1)2 | 3250(w) | 1153(m) | 1340, 1005,760 |
1610(m) |
1618(m) |
1520(m) |
1420(m) |
1340(m) |
| Cu (L2)2 | 3250(w | 1155(m) | 1343,1008,764 |
1613(m) |
1620(m) |
1520(m) |
1421(m) |
1341(m) |
| Cu (L3)2 | 3250(w) | 1157(m) | 1349,1012,768 |
1616(m) |
1622(m) |
1522(m) |
1422(m) |
1343(m) |
| Co (L1)2 | — | 1154(m) | 1343,1009,764 |
1613(m) |
1618(m) |
1537(m) |
1442(m) |
1358(m) |
| Co (L2)2 | — | 1158(m) | 1353,1015,772 |
1615(m) |
1619(m) |
1541(m) |
1444(m) |
1364(m) |
| Co (L3)2 | — | 1160(m) | 1345,1012,766 |
1610(m) |
1617(m) |
1539(m) |
1440(m) |
1360(m) |
Table -3:- Electronic spectral bands of Cu(II)and Co(II)complexes
| Complexs | 2B1g ─˃2A1g | 2B1g─˃2B2g | 2B1g─˃2Eg | 4T1g(F)─˃4A2g | 4T1g(F)─˃4T1g(P) |
| Cu(L1)2 | 719nm | 356nm | 270nm | ||
| Cu(L2)2 | 724nm | 360nm | 275nm | ||
| Cu(L3)2 | 728nm | 365nm | 280nm | ||
| Co(L1)2 | 512nm | 460nm | |||
| Co(L2)2 | 514nm | 463nm | |||
| Co(L3)2 | 516nm | 465nm |
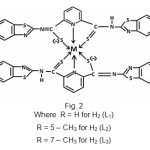 |
Figure 2 Click here to View figure |
Conclusion
The tentative structure of the complex, on the basis of above discussion were proposed as
References
- Sigel H., Martin R.B., Chem. Rev. 1982, 82, 385.
- Conlon D.A., Yasuda N.A., Catal Synth 2001, 1, 343.
- Trost B.M., Hachiya I., J. Am. Chem. Soc. 1998, 120, 1104.
- Collinson S.R., Gelbrick T., Hurshtouse M.B., Tusher J.H.R., Chem. Commun. 555 , 2001.
- Huc I., Krische M.J., Funeriu D.P., Lehan J.M., Eur. J. Inorg. Chem. 1999, 1415.
- Epperson J.D. Ming L.J., Baker G.R., Newkome G.R., J. Am. Chem. Soc. 2001, 123, 8583.
- Matsujaki M., Okable H., Tanaka S., Japan Kokai 1982, 77, 33676.
- Kumar, M., Saxena, P.N., Orient J. Chem., 2012, 28(4), 1927-1931.
- Vogel A.I., ‘’ A text book of quantitative Inorganic Analysis “ Longmans, 1964, P.P. 358 – 479.
- Lever A.B.P, J. Chem. Ed., 1968, 45(11), 711.

This work is licensed under a Creative Commons Attribution 4.0 International License.









