Influence of Steric Hindrance on The Antioxidant Activity of Some Schiff Base Ligands And Their Copper(II) Complexes
Muhammad Saleh Salga1, Ibrahim Sada1 and Abdullahi Mustapha2
1Department of Pure and Industrial Chemistry, Umaru Musa Yar’adua University, Katsina, Nigeria.
2Department of Chemistry, Federal University Dutse, PMB 7156 Jigawa State, Nigeria
DOI : http://dx.doi.org/10.13005/ojc/300410
Article Received on :
Article Accepted on :
Article Published : 20 Nov 2014
Herein we report the synthesis, characterization and antioxidant activity of the Schiff base ligands (E)-4-bromo-2-((2-piperazine-1-yl)ethylimino) methylphenol and 2,2’-(1E,1E’)-cyclohexane-1,2-diylbis(azan-1-yl-1-ylidene))bismethan-1-yl-1-ylidene)bis (4-bromophenol) and their complexes of copper (II) ion. Antioxidant activities of the copper complexes were studied by ferric reducing antioxidant power (FRAP) assay according to the procedure reported by Benzie and Strain1 which considered the reduction of ferric tripyridyl triazine complex to a ferrous complex at low pH by monitoring the change in absorption at 593 nm. The overall results showed an increased in antioxidant activity with the increase in steric crowd. Similarly, the ligands show high activity than the complexes.
KEYWORDS:Synthesis; characterization; antioxidant activity
Download this article as:| Copy the following to cite this article: Salga M. S, Sada I, Mustapha A. Influence Of Steric Hindrance on The Antioxidant Activity of Some Schiff Base Ligands And Their Copper(II) Complexes. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Salga M. S, Sada I, Mustapha A. Influence Of Steric Hindrance on The Antioxidant Activity of Some Schiff Base Ligands And Their Copper(II) Complexes. Available from: http://www.orientjchem.org/?p=5500 |
Introduction
Interest in the symmetric and asymmetric synthesis of Schiff bases has increased significantly in recent years2. Diamines are known to coordinate to metal ions as bidentate ligands and forms both symmetric and asymmetric Schiff bases3. Being cancer one of the major leading diseases causing death in industrialized countries4, as such, development of anticancer therapies is one of the fundamental goals in medicinal chemistry. It is well known that free radicals can damage protein, lipids and DNA of bio-tissues leading to the increased rates of cancer1. Fortunately, antioxidant can prevent this damage due to their free radical scavenging activity9. Hence it is of paramount importance to develop compounds with strong antioxidant activity and verify the factors affecting this biological activity. In this paper, we study the variation in antioxidant activity with increased steric crowd by measuring the activity of the Schiff base (E)-4-bromo-2-((2-piperazine-1-yl)ethylimino) methylphenol [L1] (less crowded) and 2,2’-(1E,1E’)-cyclohexane-1,2-diylbis(azan-1-yl-1-ylidene)) bismethan-1-yl-1-ylidene) bis (4-bromophenol) [L2] (more crowded) against their Cu(II) complexes. The results were compared with that of Gallic acid and Vitamin C which are employed as reference standards to ascertain the extent of antioxidant activity of the compounds under study.
Materials and Methods
Materials: 1-(2-Aminoethyl)piperazine, 5-bromosalicylaldehyde, cyclohexane-1, 2-diamine and CuCl2 salts were purchased from Sigma-Aldrich. The spectroscopic grade DMSO-d6 was obtained from Aldrich and all other solvents used were of analytical grade and used without further purifications.
Synthesis of the Schiff base L1
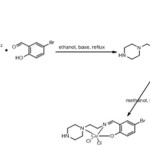
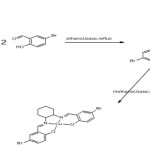
The Schiff bases and their copper complexes were prepared according to the synthetic pathway shown in scheme1 and 2 following the procedure below
A measured amount of 1-(2-aminoethyl)piperazine (0.13 g, 1mmol) was dissolved in an absolute ethanol and added drop wise to the stirred ethanolic solution of (0.2 g, 1 mmol) Bromosalicylaldehyde at room temperature. The mixture is then refluxed for 1hr to give orange solution. After evaporating the solution using rotary evaporator, red oil was formed which on addition of solid sodium perchlorate (0.1 g) produces yellow needle-like crystalline solids. Recrystallization was performed in methanol-chloroform mixture.
Synthesis complex [CuL1]
A stoichiometric amount of CuCl2 (0.17 g, 1mmol) was dissolved in methanol and added to the stirred methanolic solution (0.3g, 1mmol) of the ligand at room temperature. A green precipitate was formed, which after stirring for 5 min and settled is filtered. The filtrate is concentrated using rotary evaporator which on standing for 10 days gives black crystals. The crystals were isolated by filtration, washed with methanol and recrystallized to give pure and quality crystals.
Synthesis of the Schiff base L2
Calculated amount of cyclohexane-1, 2-diamine (0.14 g, 1 mmol) was dissolve in an absolute ethanol and added drop wise to the stirred ethanolic solution (0.2 g, 1 mmol) of Bromosalicylaldehyde at room temperature and then refluxed for 1 hr, to give yellow crystals. The crystals were removed by filtration, recrystallized in methanol and dried in a vacuum for further analysis.
Synthesis of complex [CuL2]
A stoichiometric amount of CuCl2 (0.17 g, 1mmol) was dissolved in methanol and added to the stirred methanolic solution (0.48 g, 1 mmol) of the ligand at room temperature. A green precipitate was formed after stirring for about 2 min which was allowed to settle and then filtered and dried in a vacuum.
 |
Scheme 1 Click here to View scheme |
 |
Scheme 2 Click here to View scheme |
Physical and analytical data
 |
Table 1 A Click here to View table |
Mass Spectra
The mass spectra of the ligand L1 was recorded at room temperature and showed a molecular ion peak at m/z 130 which is assignable to the decomposition of N-(Aminoethyl) piperazine and another peak at m/z 311.06(100%) which is attributed to the decomposition of the whole compound. Other peaks were observed at 313(97%), 314(14.8%), 312(14.3%). While the Ligand L2 shows molecular ion peaks at of 467(100%), 479(97%), 481(51.33%), 477(51.2%), 480(21.9%) and 478(11.5%). As shown in the table below
Table 1
| Compounds | m/z |
| L1(C13H18BrN3O) | 130, 312, 313, 314, 312, |
| L2(C20H20Br2N2O2) | 467.19, 479.9,481.9,477.9,480.9, 478, 482, 483,480, |
Infra red spectra
The IR spectra of the ligand L1 showed a broad band in the region 3400cm-1 which is assignable to N-H amine group. Appearance of this peak in the spectra of both the ligand and its Cu (II) complex indicates that the secondary amine at the extreme end of the structure is free from complexation. However, the spectrum of the ligand shows sharp absorption at 1640. cm-1 which is due to azomethine –C=N-, this absorption seems to shift to a lower frequency in the spectra of the complex which indicates the involvement –C=N- nitrogen in the coordination to metal ion 5,6. Assignment of the proposed coordination site is further supported by the appearance of medium bands at 554 cm-1 which could be attributed to ν(M-N) and 624 cm-1 for ν(M-O)7,3.
The spectra of the ligand L2 shows sharp absorption 1630.cm-1 which is assignable to –C=N- of the azomethine, this absorption drops to a lower frequency of 1630. -1591.cm-1 in the complex indicating the coordination of –C=N- to the central metal ion. Also, the proposed coordination site was supported by the appearance of band regions at 513 cm-1 for ν(M-N) and 570 cm-1 which is assignable to ν(M-O) respectively as shown in the table below
Table 2
| Compound | ν(N-H) | ν(C-H) aliphatic | ν(C=N) | ν(C-C) | ν(C-N) | ν(O-H) | ν(C-H) | ν(M-O) | ν(M-N) |
| Aromatic | aromatic | ||||||||
| L1(C13H18BrN3O) | 3421 | 2736 | 1638 | 1458 | 1176 | 3421 | 688 |
– |
– |
| CuL1(C13H18BrN3O) | 3413 | 2947 | 1635 | 1478 | 1182 | – | 694 | 624 | 554 |
| L2(C20H20Br2N2O2) | none | 2926 | 1630 | 1475 | 1184 | 3434 | 628 |
– |
– |
| CuL2(C20H20Br2N2O2) | none | 2935 | 1630 | 1455 | 1175 | – | 647 | 570 | 513 |
1H-NMR spectra
The HNMR spectra of the compound L1 was recorded in CDCl3 and the following signals were observed. N-H amine was observed at 2.17 ppm, methylene (CH2) with 1-α-N(C) C and 1-β-N-C at 2.51 ppm, the chemical shift at 4.32 ppm can be assign to aromatic alcohol while at 7.37 ppm the shift can be attributed to aromatic bromide. Azomethine was observed at 8.29 ppm.
The spectrum of the L2 shows aromatic alcohol at 3.5 ppm, methylene CH2 at 2.52 ppm, aromatic bromide at 7.25ppm and azomethine at 8.98 as shown in table 3.
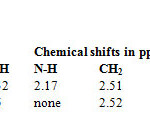 |
Table 3 Click here to View table |
UV-Visible
The UV-Visible spectra of the complexes were carried out in DMSO and their tentative assignments are as follows:
The spectra of L1 shows absorption of 3.073 at 279 nm which is assignable to π-π* electronic transition this is followed with a bathochromic shift at wavelength 303 nm and remain constant at wavelengths 312 nm, and 344 nm, this is attributed to n-π* electronic transition due to nitrogen groups found in the various part of the ligand. However, the spectra of its complex (CuL1) shows absorption of 2.552 at 274 nm which is assignable to π-π* this absorption then move to a high wavelength of 377 nm which is very close to the wavelength of 344 nm found in the ligand and can be attributable to ligand-metal charge transfer, another absorption at 636 nm was observed and can be assign to d-d electronic transition in Cu (II).
The spectra of L2 shows absorption at 267 nm which correspond to π-π* electronic transition, a bathochromic shift occur at 321 nm which is assignable to n-π* electronic transition, its CuL2 complex shows absorption at 272 nm which can be attributed to π-π* electronic transition, another absorption at 372 nm in relation to the absorption at 321 nm in the spectra of the ligand can be assignable to ligand-metal charge transfer, while absorption at 588 nm can be attributed to d-d electronic transition as shown in the table 4 below.
Table 4
|
Compound |
Wavelength |
Absorption |
Description |
|
L1 |
279 |
3.073 |
π-π* |
|
303 |
3.135 |
n-π* |
|
|
312 |
3.135 |
n-π* |
|
|
344 |
3.135 |
n-π* |
|
|
L2 |
267 |
2.916 |
π-π* |
|
321 |
3.263 |
n-π* |
|
|
CuL1 |
274 |
2.552 |
π-π* |
|
377 |
1.554 |
L-MCT |
|
|
636 |
0.144 |
d-d |
|
|
CuL2 |
272 |
2.592 |
π-π* |
|
372 |
1.418 |
L-MCT |
|
|
588 |
0.354 |
d-d |
Biological Activity
The antioxidant activity results presented in figure 13 showed that the synthesized ligands and their metal (II) complexes posses antioxidant activity. The compounds were screened for their antioxidant activity and the scavenging ability of the ligands as well as their complexes against hydroxyl radicals were tested as a function of concentration from 0-0.8μM as shown in figure 13. It can be seen that the inhibitory effects of complexes on the hydroxyl radicals are related to concentration. The scavenging activities was found to be more on the ligands than on their Cu(II) complexes and is increase with increasing steric crowding according to the following order L2>L1>CuL2>CuL1 detail was shown on the tables 5 & 6 and figures 1& 2 below.
Table 5: Antioxidant activity
|
200 |
0.263 |
0.261 |
0.256 |
0.26 |
0.144 |
|
|
400 |
0.379 |
0.41 |
0.383 |
0.391 |
0.275 |
|
|
600 |
0.52 |
0.545 |
0.585 |
0.55 |
0.434 |
|
|
800 |
0.707 |
0.703 |
0.704 |
0.705 |
0.589 |
|
|
1000 |
0.841 |
0.809 |
0.925 |
0.858 |
0.742 |
Table 6
| samples | rep1 | rep2 | rep3 | Mean | avg-blnk | frap value | |
| L2 |
0.245 |
0.239 |
0.235 |
0.240 |
0.124 |
176.7 |
|
| L1 |
0.215 |
0.23 |
0.219 |
0.221 |
0.105 |
150.5 |
|
| CuL2 |
0.182 |
0.16 |
0.175 |
0.172 |
0.056 |
80.5 |
|
| CuL1 |
0.155 |
0.137 |
0.147 |
0.146 |
0.030 |
43.3 |
|
| GA 10x |
2.227 |
2.134 |
2.135 |
2.165 |
2.049 |
2927.6 |
|
| AA 10x |
1.161 |
1.1156 |
1.234 |
1.170 |
1.054 |
1506.0 |
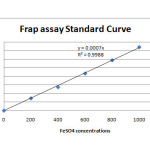 |
Figure 1 Click here to View figure |
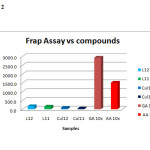 |
Figure 2 Click here to View figure |
Conclusion
The synthesized compounds showed antioxidant properties. By comparison, the ligands showed high antioxidant activity than their Cu (II) complexes. However, none of the compounds were found to have high activity than vitamin C and/or Gallic acid. L2 reveals the highest radical scavenging activity among the series whereas the compound CuL1 reveals the lowest. The different relative scavenging activity of the individual compounds against different radicals may be attributed to steric crowd, number of free hydroxyl groups and other factors such as stereo selectivity of the radicals or the solubility of these compounds in different testing systems may also affect the capacity of individual compounds to react and quench different radicals8.
Acknowledgment
The authors acknowledged the financial support from TETFUND, the Department of Pure and Industrial Chemistry where the research work is conducted and Umaru Musa Yaradua University for providing the facilities for this study.
Reference
- Benzie, F.F.; and J.J. Strain, J.J. Method Enzymol. 1999, 299:15-23
- Dreher, D.; and Junod, A. F. Eur. J. Cancer. 1996, 32: 30.
- Katsuki, T. Chem. Soc. Rev., 2004, 33, 437-444
- Nakamoto, K. Infra red and Raman spectra of inorganic and coordination compounds, New York: Wiley 3rd edition.
- Barceo-Oliver, M.; Garcia-Raso, A.; Terron, A.; Molins, E.; Prieto, M.J.; Moreno, V. Martinez, J.; Llado, V.; lopez, I.; Gutierrrez, A.; Escriba, P.V. J. Inorg. Biochem. 2007, 101:649
- Iskander, M.F.; Ei-syed, L.; Ismail, K.Z. Trans. Met. Chem. 1979, 4: 225
- Thankamony, M.; and Mohanan, K. Indian J. Chem. 2007, A46: 249
- Thomas, M.; Nair, M.K.M.; Radhakrisan, R.K. Synth.Reac. Inorg. Met.-Org. Chem. 1995, 25: 471
- Wang S.Y.; and Jiao, H. J. Agirc. Food. Chem. 2000, 48: 5677-5684
- Chen, W.J.; Guo, P.; Song, J.; Cao, W.; Bian, J. Bioorg. Med. Chem Lett. 2006, 16:3582

This work is licensed under a Creative Commons Attribution 4.0 International License.









