Design and synthesis of spiro derivatives containing a thiophene ring and evaluation of their Anti-microbial activity
Geethanjali Kanagaraju, and Arumugam Thangamani*
Department of Chemistry, Karpagam University, Coimbatore-641 021, Tamil Nadu, India.
DOI : http://dx.doi.org/10.13005/ojc/300421
Article Received on :
Article Accepted on :
Article Published : 17 Nov 2014
Synthesis of a series of novel spiro pyrrolidines has been accomplished by 1, 3-dipolar cycloaddition reaction of azomethine ylide generated from phenylalanine and isatin with dipolarophiles in good yield. The reaction proceeded with high regio and stereoselectivity. The products have been characterized by elemental analyses and spectroscopic techniques, namely IR, 1H NMR and 13C NMR spectroscopies. Single crystal analysis of compounds 4k 2D-NMR analysis of compound 4k confirmed the structures of spiropyrrolidine derivatives. These compounds were evaluated for their antimicrobial activity. Most of the synthetic compounds exhibited good activity against microorganisms.
KEYWORDS:1; 3 dipolar cycloaddition; azomethine ylide; dipolarophiles; spiro pyrrolidines; antimicrobial activity
Download this article as:| Copy the following to cite this article: Kanagaraju G, Thangamani A. Photochemical treatment as an alternative to improve the quality of wastewater after advanced primary treatment. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Kanagaraju G, Thangamani A. Photochemical treatment as an alternative to improve the quality of wastewater after advanced primary treatment. Orient J Chem 2014;30(4). Available from: http://www.orientjchem.org/?p=5400 |
INTRODUCTION
Heterocyclic compounds predominantly five and six membered ring compounds have occupied a massive place among varied categories of organic compounds for their various biological activities.1 Among a wide variety of heterocycles that are explored for developing pharmaceutically necesaary molecules, isatin have played an vital role in medicinal chemistry.2-4 A number of them have received significant attention as potential antimicrobial agents. 5-6 Indol-2, 3-diones (Isatin) represent an important class of medicinal agents and their importance has steadily increased over the past few decades as result of their potentiality towards a variety of organic reactions together with cycloaddition reactions.7Moreover, spiro [indole-pyrrolidine] ring systems acquired a special place within the heterocyclic field that have additionally drawn much attention attributable to their necessary pharmacological properties. 8
The 1,3-dipolar cycloaddition reaction provides a straightforward and direct entry into a vairety of five-membered heterocyclic compounds, such as pyrrolidines, pyrrolines, and pyrroles. This reaction has also been employed for the construction of spiro compounds, and by this fashion several spiro [pyrrolidine-2, 3’-oxindoline] derivatives have been synthesized.9-10 The azomethine ylide represents one of the foremost reactive and versatile categories of 1,3- dipoles and is instantly concerned trapped by a range of dipolarophiles forming substituted pyrrolidines.11 1,3-dipolar cycloaddition reactions of azomethineylides has been well developed, and also the reactions proceed with high regio- and stereoselectivity12,13. Thiophene and its derivatives are the significant class of heterocyclic compounds possessing broad biological activities, such as anti-inflammatory14, analgesic14, antioxidant15, antitubercular,16 antidepressant,17 sedative,18 antiamoebic,19 oral analgesic,20 anti-metabolite,21 and antineoplastic properties.22
For a extended analysis of the regio-chemistry in 1,3-dipolar cycloaddition reaction and also the bioactivity of spiro [pyrrolidine-2,3′-oxindoline] compounds, three varieties of trapping agents like dipolarophiles, diketone, α- amino acid, were introduced during this reaction to synthesis a series of spiro [pyrrolidine-2,3′-oxindole] derivatives, with that the bioactivity on anti-microbial activity was evaluated. In continuation of our research interests concerning the economical assembly of spiro-oxindoles ,we wish to report herein a 1,3-dipolar cycloaddition reaction involving enone segment (E)-3-aryl-1-(thiophen-2-yl)-prop-2-en-1-one to synthesize spiro oxindoles. To the most effective of our information , this is often the primary report on (E)-3-aryl-1-thiophen-2-yl)-prop-2-en-1-one employed as dipolarophile in cycloaddition of azomethine ylides to synthesize functionalised spiro-oxindoles.23 This reaction is regioselective with the addition of the electron rich carbon of the dipole to the β-carbon of 4a-4k and stereoselective affording only one isomer in good-to-excellent yields, albeit four stereocenters are present in these cycloadducts. The atom-economy of the reaction is also very high, viz. 80–97% as water and carbon dioxide alone are generated as waste.
Experimental
Apparatus and analysis
IR spectra were recorded in AVATAR-330 FT-IR spectrophotometer.1H NMR spectra were recorded at 400 MHz on BRUKER DRX spectrophotometer using CDCl3 as solvent and TMS as an internal standard. 13C NMR spectra were recorded at 100 MHz on BRUKER DRX spectrometer in CDCl3 Microanalysis was performed on Heraeus Carlo Erba 1108 CHN analyzer. The melting points were recorded in open capillaries and are uncorrected. The following abbreviations were used to indicate the peak multiplicity s-singlet, d-doublet, t-triplet, q-quartet, and m-multiplet. The various substituted (E)-3-aryl-1-(thiophen-2-yl) prop-2-en-1-ones 2a-2k were synthesized in accordance with the literature23.
General procedure for preparation of the (E)-3-aryl-1-(thiophen-2-yl) prop-2-en-1-ones ( 2a-2k)
To an ethanolic solution (30 mL) of 2-acetyl thiophene (1 mmol) and substituted benzaldehyde (1 mmol), aqueous sodium hydroxide (5 mmol) was added dropwise with mechanical stirring over 10 min. Stirring was continued for the time indicated in (Table 2) After completion of the reaction, the crude product was isolated by filtration and washed with water, dried, and recrystallized from ethanol23.
General procedure for the synthesis of thiophenyl grafted spiropyrrolidines bearing oxindole system (4a-4k)
A mixture of isatin (2) (1 mmol), phenyl alanine (1 mmol) and (E)-3-aryl-1-(thiophen-2-yl) prop-2-en-1-ones (3a-3k) (1 mmol) in methanol (20 mL) was refluxed with stirring for the appropriate time (see Table 2). The reaction mixture was cooled to room temperature. The solid precipitated from the reaction was filtered and recrystallized from ethanol to afford crystalline products.
5′-benzyl -3′-(thiophene-2-carbonyl) 4′-phenyl spiro[indoline-3,2′-pyrrolidin]-2-one.(4a)
Yield 92%, Melting point 198-201˚C,IR(KBr, cm-1) 1714 (thiophenyl carbonyl), 1653 (oxindole carbonyl).1H NMR (CDCl3) δ: 2.70 (H5-CH2) (dd, 1H, J = 7.6&14 Hz), 2.99 (H5-CH2) (dd, 1H, J = 3.6&14 Hz), 3.88 (H4) (t,1H,J=10.8Hz), 4.23-4.29 (H5)( m,1H) 4.43(H3) (d, 1H,J=10.8Hz), 6.50 (1H,d, J = 7.6 Hz), 6.81 (dd, 1H, J = 4 & 4.8 Hz), 6.89-6.91 (m ,2H) 6.99-7.03 (m ,1H), 7.16-7.20 (m ,4H), 7.32-7.36 (m ,4H), 7.53-7.55 (m ,2H), 7.98 (-NH of oxindole ring 13C NMR (CDCl3) δ:38.7, 52.8, 64.5, 64.8, 69.3109.3, 123.2, 125.9, 126.4, 127.1, 127.6, 128.4, 128.5, 128.8, 129.1, 129.3, 129.4, 131.2, 134.2, 37.9, 139.2, 139.7, 144.6; 182.0 (oxindole carbon) 189.0 (thiophenyl carbon),Anal. Calcd for C29H24N2O2S: C, 74.97; H, 5.21; N, 6.03; O, 6.89 ;S, 6.90. Found: C, 74.99; H, 5.20; N, 6.08 %.
5′-benzyl3′-(thiophene-2-carbonyl)-4′-(4-methylphenyl)-spiro[indoline-3,2′-pyrrolidin]-2-one (4b)
Yield 90%, Melting point 188-192˚C,IR(KBr, cm-1) 1712 (thiophenyl carbonyl), 1633(oxindole carbonyl).1H NMR (CDCl3) δ: 1.65 (-NH of pyrrolidine ring) (s,1H), 2.69 (H5-CH2) (dd, 1H, J = 7.8 & 13.8 Hz) 2.99(H5-CH2) (dd, 1H, J = 2.4 &12.8 Hz), 3.85( H-4) (t,1H,J =10.8Hz), 4.40 (H-3) (d, 1H,J =10.8Hz), 4.20- 4.25( H5) ( m,1H) 6.50 (1H,d, J = 8 Hz), 6.80 (t, 1H, J = 4 .4Hz), 6.88-6.91(m ,2H) 6.98-7.14(m ,1H), 7.16 (d ,2H J =8.4Hz), 7.19-7.21(m ,3H), 7.24-7.27(m ,2H),7.42(d ,2H,J =8Hz),-NH of oxindole ring, 7.78,(s,1H), 13C NMR (CDCl3) δ:15.8, 33.3, 47.2, 59.1, 59.5, 63.9, 104.0, 117.9, 120.7, 121.1, 122.3,1 23.2, 123.9, 124.2, 124.2, 124.3, 126.0, 128.9, 139.5, 176.5, (oxindole carbon ) 183.8 (thiophenyl carbon); Anal. Calcd for C30H26N2O2S: C, 75.29; H, 5.48; N, 5.85,O, 6.69,S,6.70. Found: C, 75.33; H, 5.45; N, 5.90 %.
5′-benzyl3′-(thiophene-2-carbonyl)-4′-(4-methoxyphenyl)-spiro[indoline-3,2′-pyrrolidin]-2-one(4c)
Yield 92%, Melting point 198-201˚C,IR(KBr, cm-1) 1712 (thiophenyl carbonyl), 1639 (oxindole carbonyl).1H NMR (CDCl3) δ: 1.26 (-NH of pyrrolidine ring) (s,1H), 2.68 (H5-CH2) (dd, 1H, J = 7.6&14 Hz), 2.99 (H5-CH2) (dd, 1H, J = 3.2 &14 Hz), 3.78 (O-CH3) (s,3H), 3.83 (H4) (t,1H,J =10.4Hz), 4.17-4.23 (H5) ( m,1H) 4.37 (H3) (d, 1H,J =10.8Hz), 6.51 (1H,d, J = 8 Hz), 6.81 (t, 1H,J = 4.2Hz), 6.87-6.90 (m ,4H) 6.99-7.02 (m,1H),) 7.16-7.20 (m,4H),7.23-7.27(m,2H) 7.33(d ,3H J =4.4Hz), 7.45(d ,3H, J =8.8), 7.78,(-NH of oxindole ring) (s,1H),13C NMR (CDCl3),38.7, 52.0, 55.2, 64.4, 64.7, 69.2, 109.2, 114.2, 123.1, 125.9, 126.4, 127.6, 128.4, 129.1, 129.4, 129.5, 131.1, 131.2, 134.1, 138.0, 139.7, 144.7, 158.7, 181.9, (oxindole carbon), 189.2 (thiophenyl carbon). Anal. Calcd for C30H26N2O3S: C, 72.85; H, 5.30; N, 5.66; O, 9.70; S, 6.48. Found: C, 72.88; H, 5.28; N, 5.63 %.
5′-benzyl-3′-(thiophene-2-carbonyl)-4′-(4-methylthiophenyl)-spiro[indoline-3,2′-pyrrolidin]-2-one(4d)
Yield 91%, Melting point 199-202˚C, IR(KBr, cm-1) 1712 (thiophenyl carbonyl), 1633(oxindole carbonyl).1H NMR (CDCl3) δ: 1.65, -NH of pyrrolidine ring (s,1H), ), 2.46 (-SCH3) (s,1H) 2.69 (H5-CH2) (dd, 1H, J = 7.6 &14 Hz), 2.98 (H5-CH2) (dd, 1H, J = 3.2 &14 Hz), 3.83 (H4) (t,1H,J =10.6Hz), 4.20-4.25 (H5) (m,1H) 4.37 (H3) (d, 1H, J=10.8Hz),6.51(d,1H J=8Hz), 6.78-6.99 (m,1H), 6.86-6.90 (m,1H), 6.96 (m,1H J =8.8Hz), 6.97-7.0 (m,1H), 7.15-7.20 (m,3H), 7.22-7.28 (m,2H), 7.30-7.33 (m,3H), 7.32-7.34 (m,2H), 7.38-7.42 (m,2H), 7.45 (d,1H, J = 8.8Hz), 7.46 (-NH of oxindole ring,), (s,1H). 13C NMR (CDCl3) 38.7, 52.3, 64.4, 64.7, 69.1, 15.9, 109.1, 115.2, 123.2, 125.9, 126.4, 127.2, 127.6, 128.4, 129.0, 129.1, 129.4, 129.5, 131.3, 134.2, 136.1, 137.0, 137.9, 139.6, 144.9, 181.6,(Oxindole carbon) 189.0 (Thiophenyl carbon). Anal.Calcd for C36H30N2O3S: C, 75.76; H, 5.30; N, 4.91; O, 8.41; S, 5.62. Found: C, 75.74; H, 5.26; N, 4.95 %.
5′-benzyl-3′-(thiophene-2-carbonyl)4′-(4-nitrophenyl)-spiro[indoline-3,2′-pyrrolidin]-2-one (4e)
Yield 85%, Melting point 198-201˚C,IR(KBr, cm-1) 1714 (thiophenyl carbonyl), 1637 (oxindole carbonyl).1H NMR (DCl3) δ: 1.26 (s,1H), 2.78 (H5-CH2) (dd, 1H, J = 7.4 &13.8 Hz), 2.93 (H5-CH2) (dd, 1H, J = 3.8 &13.8 Hz), 4.01 (H4) (t,1H,J=10.4Hz), 4.33-4.34 (H5) ( m,1H) 4.38 (H3) (d, 1H,J=10.4Hz), 6.52(1H,d, J = 7.6 Hz) , 6.82 (t, 1H, J = 4.2Hz), 6.89-6.96(m ,2H) 7.03 (t ,1H),7.13 (d,2H, J=7.2Hz) 7.17-7.24 (m,4H),7.32(d ,1H J=3.2 Hz), 7.38 (d ,1H, J =4.8), 7.67 (d, 2H J=8.4Hz),7.83-7.90 (m,1H) 13C NMR (CDCl3) δ:39.1, 52.8, 64.7, 64.8, 69.3, 109.5, 123.3, 123.9, 124.2, 125.8, 126.6, 129.3, 132.4, 134.6, 134.7, 137.3, 139.8, 147.1, 147.4, 181.5 (oxindole carbon), 185.5, (thiophenyl carbon). Anal. Calcd for C29H23N3O4S: C, 68.35; H, 4.55; N, 8.25; O, 12.56; S, 6.29. Found: C, 68.39; H, 4.52; N, 8.22 %.
5′-benzyl-3′-(thiophene-2-carbonyl)4′-(4-fluorophenyl)-spiro[indoline-3,2′-pyrrolidin]-2-one(4f)
Yield 94%, Melting point 198-200˚C,IR (KBr, cm-1) 1714 (thiophenyl carbonyl), 1635 (oxindole carbonyl).1H NMR (CDCl3) δ: 2.71 (H5-CH2) (dd, 1H, J = 7.4&13.8 Hz), 2.96 (H5-CH2) (dd, 1H, J = 3.2 &14 Hz), 3.87 (H4) (t,1H,J =10.8 Hz), 4.20-4.25 (H5)( m,1H) 4.35 (H3) (d, 1H,J =10.8Hz), 6.50 – 6.54 (m,1H), 6.80-6.84(m,1H),6.90 – 6.91- (m,2H),7.00-7.04 (m,3H) 7.14 – 7.20 (m,3H), 7.23 – 7.26 (m,2H), 7.32-7.36 (m,2H) 7.47-7.50 (m,2H), 7.99, (-NH) (s,1H),13C NMR (CDCl3), 40.1, 53.4, 65.9, 66.1, 70.5, 110.7, 117.0 , 124.5, 127.5,127.8,129.4, 129.8, 130.6, 130.7, 131.3, 131.4, 135.6, 136.2, 136.3, 139.2, 141.1,145.8,163.0,129.5,1 31.1, 131.2, 134.1, 138.0, 139.7, 144.7, 158.7, 181.9, (oxindole carbon), 189.2 (thiophenyl carbon). Anal. Calcd for C30H26N2O3S: C, 72.85; H, 5.30; N, 5.66; O, 9.70; S, 6.48. Found: C, 72.83; H, 5.28; N, 5.68%.
5′-benzyl-3′-(thiophene-2-carbonyl)4′-(4-chlorophenyl)-spiro[indoline-3,2′-pyrrolidin]-2-one (4g)
Yield 93%, Melting point 194-196˚C,IR(KBr, cm-1) 1710 (thiophenyl carbonyl), 1643 (oxindole carbonyl).1H NMR (CDCl3) δ: 2.71 (H5-CH2) (dd, 1H, J = 5.4&11.8 Hz), 2.97 (H5-CH2) (dd, 1H, J = 6.8 &14 Hz), 3.85 (H4) (t,1H,J =10.8Hz), 4.20-4.26 (H5)( m,1H) 4.35 (H3) (d, 1H,J =10.4Hz), 6.08 (1H,d, J = 14.4 Hz), 6.49 (d, 1H, J = 7.6Hz), 7.07-7.10(m ,2H) , 7.13-7.19(m ,1H), 7.38(d,3H,J =4.8Hz), 7.53-7.55(m ,2H), 7.59(d ,2H, J =8.4Hz), 7.59-7.70(m ,2H), 7.85,(s,1H), 13C NMR (CDCl3)δ: 109.2, 122.9, 125.8, 125.9, 126.8, 127.6, 128.5, 128.6, 128.9, 129.1, 129.4, 130.4, 131.4, 134.3, 134.5, 137.7, 139.6, 141.4,143.9, 180.8; (oxindole carbon) 187.7, (thiophenyl carbon). Anal. Calcd for C29H23ClN2O2S: C, 69.80; H, 4.65; Cl, 7.10; N, 5.61; O, 6.41; S,6.43. Found: C, 69.84; H, 4.67; N, 5.63 %.
5′-benzyl-3′-(thiophene-2-carbonyl)4′-(3-chlorophenyl)-spiro[indoline-3,2′-pyrrolidin]-2-one(4h)
Yield 86%, Melting point 195-197˚C,IR(KBr, cm-1) 1710 (thiophenyl carbonyl), 1625 (oxindole carbonyl).1H NMR (CDCl3) δ: 2.24 (-NH of pyrrolidine ring, )(s,1H) , 2.72 (H5-CH2) (dd, 1H, J = 7.6 &14 Hz), 2.97 (H5-CH2) (dd, 1H, J = 3.8 &13.8 Hz), 3.85 (H4) (t,1H,J=10. 6Hz), 4.22-4.27 (H5) ( m,1H), 4.37 (H3) (d, 1H,J =10.8Hz), 6.52 (d,1H J = 7.6Hz), 6.83,(dd,1H, J = 4 & 4.8Hz), 6.91-6.96 (m,2H), 7.00-7.15 (m,2H),7.16-7.18,(m,2H) ,7.19-7.21 (m,2H),7.23-7.28 (m,3H) 7.34 (dd,1H J =0.8 & 4.4Hz), 7.37 (dd,1H J =5 & 1Hz), 7.40-7.42 (m,1H) 7.50-7.51(m,1H), 7.71(-NH of oxindole ring) (s,1H),13C NMR (CDCl3), 38.8, 52.5, 64.4, 64.6, 69.2, 109.3, 123.2, 125.8, 126.5, 126.9, 127.4 127.6, 128.4, 128.6, 129.1, 129.2, 129.4, 130.0, 131.3, 134.4, 134.6, 137.7, 139.7, 141.5, 144.4,181.6, (Oxindole carbon),188.8, ,(Thiophenyl carbon). Anal. Calcd for C29H23ClN2O2S: C, 69.80; H, 4.65; Cl, 7.10; N, 5.61; O, 6.41; S, 6.43. Found: C, 69.86; H, 4.69; N, 5.66 %.
4′-(3-bromophenyl)-5′-benzyl-3′-(thiophene-2-carbonyl)spiro[indoline-3,2′-pyrrolidin]-2-one (4i)
Yield 84%, Melting point 192-195˚C,IR(KBr, cm-1) 1710 (thiophenyl carbonyl), 1631(oxindole carbonyl).1H NMR (CDCl3) δ: 2.25, NH of pyrrolidine ring(s,1H), 2.72 (H5-CH2) (dd, 1H, J = 7.6&14 Hz), 2.96 (H5-CH2) (dd, 1H, J = 3.8 &13.8Hz), 3.84 (H4) (t,1H,J =10.8Hz),4.21-4.27 (H5)(m,1H) 4.36 (H3) (d, 1H, J =10.8Hz), ), 6.51 (d,1H J =7.6Hz), 6.84,(dd,1H,J= 3.8 & 5.0 Hz) 6.89-6.91 (m,2H), 7.00 – 7.04 (m,1H) ,7.14 – 7.17, (m,2H) ,7.18 (d,1H J=3.6Hz),7.20(d,1H,J=2.8Hz),7.23, (d,1H J=1.2Hz), 7.25 (d,1H J=6Hz). 7.65 (-NH of oxindole ring), (s,1H), 7.34-7.38 (m,3H), 7.44-7.47,(m,1H),7.65-7.66,(m,1H)13C NMR (CDCl3) δ: 38.9, 52.4, 64.4, 64.9, 69.1, 109.2, 122.9, 123.2, 125.9, 126.5, 127.4, 127.7, 128.5, 129.1, 129.3, 129.4, 130.3, 130.4, 131.4, 131.5, 134.4, 137.7,139.6, 141.8, 144.4,181.4 (Oxindole carbon),188.8,(Thiophenyl carbon). Anal. Calcd for C29H23BrN2O2S: C, 64.09; H, 4.27; Br, 14.70; N, 5.15; O, 5.89; S, 5.90. Found: C, 64.14; H, 4.30; N, 5.20%.
4′-(4-Bromophenyl)-5′-benzyl-3′-(thiophene-2-carbonyl)spiro[indoline-3,2′-pyrrolidin]-2-one (4j)
Yield 88%, Melting point 190-192˚C,IR(KBr, cm-1) 1714 (thiophenyl carbonyl), 1643 (oxindole carbonyl).1H NMR (CDCl3) δ: 2.63 (H5-CH2) (dd, 1H, J = 7.6& 14 Hz), 2.89 (H5-CH2) (dd, 1H, J = 3.2 & 13.6 Hz), 3.77 (H4) (t,1H,J =10.8Hz), 4.12-4.17 (H5) ( m,1H) 4.27 (H3) (d, 1H,J =10.4Hz), 6.43 (1H,d, J = 7.6 Hz), 6.49 (d, 1H, J = 7.6Hz), 6.74 (t, 1H, J = 4.4Hz), 6.82 (d,2H J = 6.8Hz) 6.92-6.96 (m ,1H) 7 .07 (d ,2H), 7.13-7.19 (m ,1H), 7.19 (m,2H) 7.25 (d ,1H J =3.6 Hz), 7.32 (d ,2H, J =8.4Hz), 7.38 (d,2H J =8.4Hz), 7.21 ,(s,1H), 13C NMR (CDCl3) δ: 37.7, 51.2, 63.4, 63.6, 68.2, 108.3, 120.0, 122.2, 124.8, 125.5, 126.6, 127.4, 128.1, 128.3, 128.4, 129.2, 130.3, 130.9, 133.3, 136.7, 137.3, 138.7, 143.4,180.8 (oxindole carbon),187.8 (thiophenyl carbon). Anal. Calcd for C29H23BrN2O2S: C, 64.09; H, 4.27; Br,14.70, N, 5.15,O,5.89,S,5.90. Found: C, 64.13; H, 4.32; N, 5.19%.
4′-(4-benzyloxyphenyl)-5′-benzyl-3′-(thiophene-2-carbonyl)spiro[indoline-3,2′-pyrrolidin]-2-one (4k)
Yield 85%, Melting point 190-194˚C,IR(KBr, cm-1) 1712 (thiophenyl carbonyl), 1608 (oxindole carbonyl).1H NMR (CDCl3) δ: 1.26,(s,1H), 2.69 (H5-CH2) (dd, 1H, J = 7.6 &14 Hz), 2.99 (H5-CH2) (dd, 1H, J = 3.2 &14 Hz), 3.83 (H4) (t,1H,J =10.6Hz), 4.18- 4.23 (H5) ( m,1H) 4.37 (H3) (d, 1H, J =10.8Hz), 5.02 (-O-CH2) (s,2H), ), 6.51 (d,1H J = 8Hz), 6.78-6.99 (m,1H), 6.86-6.90 (m,1H),6.96,(m,1H J = 8.8Hz), 6.97-7.0 (m,1H), 7.15-7.20 (m,3H), 7.22-7.28 (m,2H), 7.30-7.33 (m,3H), 7.32-7.34 (m,2H) 7.38-7.42 (m,2H), 7.45 (d,1H, J = 8.8Hz), 8.1(-NH),(s,1H), 13C NMR (CDCl3), 38.7, 52.1, 64.4, 64.7, 69.3, 70.0, 109.4, 115.2, 123.1, 125.8, 126.4, 127.5, 127.6, 127.9, 128.4128.5, 129.1, 129.4, 129.5, 130.3, 131.4, 134.1, 137.1, 138.0, 139.8, 144.7, 158.01, 82.1,(Oxindole carbon) 189.2 (Thiophenyl carbon) . Anal. Calcd for C36H30N2O3S: C, 75.76; H, 5.30; N, 4.91; O, 8.41; S, 5.62. Found: C, 75.78; H, 5.28; N, 4.97%.
X-ray crystallography
X-ray data collection, structure determination and refinement of compound 4k single crystals suitable for diffraction were obtained by the slow evaporation of a solution of the compound in methanol. The orange colour crystal of the compound 4k having appropriate dimensions of 0.100 x 0.160 x 0.250 mm was mounted on a glass fiber with epoxy cement for the X-ray crystallographic study25. Bruker axs kappa apex2 CCD diffractometer equipped with graphite monochromated Mo Kα (λ = 0.71073 Å) radiation was used for the measurement of data. The collected data were summarised using the SAINT program and structural refinement was carried out by Full-matrix least-squares on F^2 (SHELXL-97),.26 Molecular graphics employed include ORTEP and PLATON.27 The ORTEP view of the compound with atomic numbering is shown in Fig. 2.
Results and discussion
Chemistry
In the present investigation, the 1,3-dipolar cycloaddition of azomethine ylides, generated insitu via decarboxylative condensation of substituted dipolarophiles (3a-k) , isatin(4) and phenylglycine (5) in methanol afforded novel spirooxindole pyrrolidine 6a-k in high yields. (Scheme : 1)
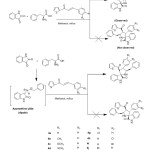 |
Scheme1: Synthesis of thiophenyl grafted spiropyrrolidines. Click here to View scheme |
Different choice of solvents such as methanol, ethanol, dimethyl sulfoxide and acetonitrile and toluene were used to optimise the reaction condition. Among these, methanol is the appropriate solvent to relinquish high yields at minimum time and also purification of the product by precipitation is much easier when compared to dimethyl sulfoxide and toluene. Ultimately, this reaction was performed by heating an equimolar mixture of substituted chalcones (3a-k), (2) in methanol under reflux for 3-4 h. After completion of the reaction, as indicated by TLC, the reaction mixture was poured into ice-water; the resulting solid was filtered off and washed with ethanol to obtain pure spirooxindole pyrrolidine derivatives 6a-k in 82-94% yields in region-and stereocontrolled manner. (Table: 1)
 |
Table1: Reaction optimization for the formation. Click here to View table |
The structure and regiochemistry of the cycloadducts were characterized by spectroscopic data The results are summarised in (Table 2).
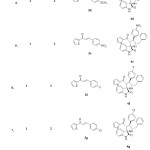 |
Table2: Synthesis of oxindole fused thiophenyl grafted spiropyrrolidines 4a-4k. Click here to View table |
The two possible regio approaches of 1,3-dipole and dipolarophile were shown in path A and B in Scheme 3. Two π-electrons of the dipolarophile and four electrons of the dipole participate in a concerted, pericyclic shift. The addition is stereo conservative (suprafacial) and the reaction is therefore a [2S+4S] cycloaddition. This reaction occurs through the interaction between HOMO of azomethine ylide and LUMO of the alkene. The regioselective product which cyclises solely via path B instead of path A. But, two diastereomers are doable for this regioselective product which were shown in path X and Y in Scheme 3. The transition state fashioned by path X stabilised by secondary orbital interaction between carbonyl groups of 1,3-dipole and dipolarophile, whereas no such secondary orbital interaction is feasible in path Y, therefore the reaction proceeds via path X and only resulting in regio- and diastereoselective spirooxindole-pyrrolidine products.
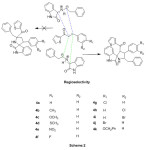 |
Scheme2: Mode of approach of regioselectivity Click here to View scheme |
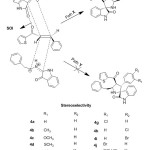 |
Scheme3: Mode of approach of stereoselectivity. Click here to View scheme |
The structures of all the products are in good agreement with their 1D and 2D NMR spectroscopic data as illustrated for 4k
The IR spectrum of spiro-pyrrolidine 4k, showed peak at 1712 cm-1 due to thiophenyl carbonyl whereas the oxindole ring carbonyl resonated at 1608 cm-1. 1H NMR spectrum of 4k displayed triplet at δ 3.83 ppm (J =10.6 Hz) due to benzylic proton (H-4) of pyrrolidine ring. The pyrrolidine ring proton of C-3 which is attached to the thiophenyl moiety appeared as a doublet at δ 4.37 ( J = 10.8Hz). The H-5 proton of pyrrolidine moiety which is attached to the phenyl alanine moiety resonated as multiplet at δ 4.23 -4.18 ppm. Two doublets of doublets at δ 2.69 and 2.99 ppm were attributed to CH2 Phdue to the diastereostopic impact of C-5. The aromatic protons appeared as multiplets in the region of δ 6.43–7.38ppm. . The –NH proton of the oxindole moiety appear as a singlet at δ 8.10 ppm. The off-resonance decoupled 13C NMR spectra of 4k added conclusive support to the proposed structure. The spiro carbon resonates at δ 69.3 ppm. The oxindole carbonyl and thiophenyl carbonyl carbon resonated at δ 182.1 and 189.2. ppm respectively. These ascertained chemical shift values confirm the proposed structure. This is often supported by their DEPT 135 method, where the spiro carbon did not show any predictable peak. The COSY experiment allowed the complete assignment of the hydrogens (4k) (Fig. 1). The doublet at 5.06 ppm (J = 10.4 Hz) reveals cross peak with the triplet focused at 4.20 ppm (J = 10.6 Hz) and this triplet shows cross peak with the doublet in the region of 4.52 ppm. This clearly indicates that doublet is attributable to pyrrolidine ring proton at C-3 which is attached to the thiophenyl moiety and the triplet is due to pyrrolidine ring proton at C-4 which is attached to the phenyl moiety.
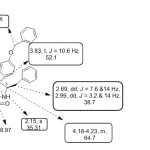 |
Figure 1 Click here to View figure |
In order to elucidate these assignments, 1H-13C COSY spectrum of compound 4k was also recorded and shown in (Fig. 2). From the 1H-13C COSY spectra the signals of all carbons containing hydrogen could be assigned unambiguously (Fig.1) The signal at 64.4 ppm showed the correlation with the pyrrolidine ring proton of C-3 at 4.37 ppm. The signal at 52.1 ppm showed a cross peak with pyrrolidine ring proton of C-4 which is attached to the phenyl moiety exhibit a triplet at 3.83 ppm. This clearly confirms that this signal is due to the C-4 carbon. From proton chemical shifts of C-2 and C-5 the corresponding carbon signal observed at 69.3 and 64.7 ppm are due to C-2 and C-5 carbons respectively in 4k.
 |
Figure 2: Design and synthesis of spiro derivatives containing a thiophene ring and evaluation of their Anti-microbial activity |
The above assignments are further confirmed by HMBC experiment (4k), The doublet at 4.37 ppm showed HMBC strong correlation with thiophenyl carbonyl (182.18 ppm) and a weak correlation with oxindole carbonyl carbon. A triplet at 3.83 ppm showed correlation with C-2’’’, C-6’’’(129.49 ppm) and C-3 (64.46 ppm). One of the methylene proton (–CH2Ph) at 2.99 (dd) which showed HMBC correlation with C-2’’’’ and C-6’’’’ (128.59 ppm). A singlet at 5.02ppm correlates with C-2’’’’’ and C-6’’’’’ (126.43 ppm).
Antimicrobial activity
All the synthesized compounds were screened for their antibacterial activity against Echerichia coli, Enterobacter aerogens Shigella flexneri,Salmonella typhimumium bacterial strains and antifungal activity against Candidaalbicans, Aspergillus niger fungal strains.The agar well diffusion method was followed to determine the antimicrobial activity. For agar well diffusion method24.Antimicrobial susceptibility was tested on solid Agar-Agar media in Petri plates. For bacterial assay nutrient agar(NA) was used for developing surface colony growth. Prepared media was then sterilized by autoclaving the media at (121˚C) for 15 min. NA plates were swabbed (sterile cotton swabs) with 8 hr old- broth culture of respective bacteria. Wells (10 mm diameter and 2cm a part) were made in each of these plates using sterile sork borer. Then a DMSO solution of test samples of different concentrations were added using sterile syringe into the wells and allowed to diffuse at room temperature for 24hrs.The plates were incubated at 37˚C for 18 -24 hrs.
The standard antimicrobial drugs (1mg/ml) Ampicillin (for bacterial assay) and (1mg/ml) Fluconazole (for fungal assay) were used as positive control. Triplicates were maintained and the experiments were repeated thrice for each replicate the readings were taken in three different fixed directions and the average values were recorded.
The antimicrobial activity was determined by measuring the diameter of the zone showing inhibition (mm), thereby the zones were precisely measured with the aid of Vernier Calipers (precise 0.1mm).The growth inhibition was calculated with reference to the positive control.
Table3: Antimicrobial activity of compounds.
|
Zone of inhibition in mm Quantity in mg/ml |
|||||||||||||||
| Sample | Echerichia coli | Enterobacter aerogens | Shigella flexneri | Salmonella typhimumium | Candida albicans | Aspergillus niger | |||||||||
|
50 |
10 |
50 |
100 |
50 |
100 |
50 |
100 |
50 |
100 |
50 |
100 |
||||
|
4a |
16 |
18 |
09 |
10 |
10 |
11 |
08 |
10 |
10 |
11 |
13 |
15 |
|||
|
4b |
18 |
19 |
13 |
14 |
06 |
07 |
09 |
11 |
10 |
10 |
13 |
15 |
|||
|
4c |
15 |
16 |
13 |
14 |
14 |
15 |
11 |
12 |
10 |
12 |
08 |
09 |
|||
|
4d |
09 |
10 |
08 |
09 |
07 |
08 |
06 |
07 |
05 |
06 |
07 |
08 |
|||
|
4e |
09 |
10 |
09 |
10 |
09 |
11 |
09 |
10 |
09 |
10 |
09 |
10 |
|||
|
4f |
21 |
22 |
19 |
20 |
16 |
18 |
19 |
20 |
19 |
20 |
19 |
20 |
|||
|
4g |
17 |
19 |
17 |
18 |
16 |
17 |
18 |
19 |
17 |
18 |
17 |
18 |
|||
|
4h |
17 |
18 |
14 |
15 |
15 |
16 |
11 |
12 |
15 |
16 |
17 |
18 |
|||
|
4i |
20 |
21 |
18 |
19 |
17 |
18 |
18 |
19 |
15 |
16 |
15 |
17 |
|||
|
4j |
20 |
21 |
18 |
19 |
17 |
19 |
17 |
18 |
19 |
20 |
18 |
19 |
|||
|
4k |
23 |
24 |
25 |
26 |
26 |
27 |
19 |
20 |
20 |
21 |
19 |
20 |
|||
|
Ampicillin |
27 |
32 |
30 |
32 |
28 |
31 |
25 |
27 |
– |
– |
– |
– |
|||
|
Fluconazole |
– |
– |
– |
– |
– |
– |
– |
– |
25 |
28 |
24 |
27 |
|||
All the synthesized compounds have been evaluated for their anti microbial activity against against Echerichia coli, Enterobacter aerogens, Shigella flexneri, Salmonella typhimumium Candida albicans, Aspergillus niger using Agar-Agar well diffusion method.The results were compared with the standard.
It is fascinating to notice that almost all the spiro derivatives exhibited some degrees of inhibition zones at 50 and 100µg dose levels. The compounds containing electron withdrawing substituent such as halogen atom favours antimicrobial activities. Furthermore the position of the substituent on the phenyl ring significantly influenced anti microbial activity, with an activity order of p-F>p-Br>m-Br>p-Cl>m-Cl derivatives. All these compounds are equally more potent against microbial strains; this again reveals that the importance of electronic effects of the substituent present in the aromatic rings in enhancing the antimicrobial activities.
Conclusions
In conclusion, we have developed an efficient synthesis of spiropyrrolidine derivatives by one-pot, three-component reaction of azomethine ylides generated from isatin with phenylalanine. This methodology has the benefits of operation simplicity, high atom economy, good-to-excellent yields in short reaction times, easy workup, delicate reaction and catalyst-free conditions. The reactions proceed with excellent chemo-regio-and stereoselectivity. Most of the synthesized compounds showed good antimicrobial activity against all the selected human pathogens. In particular, the compounds 4k, 4f, 4i and 4j exhibited the best antibacterial activity against all the microbial strains.
REFERENCES
- Chavan, P., Mane, A. S., Shingare, M. S. Indian J. Chem. 2001, 40B, 339.
- Mogilaiah, K., Ramesh Babu, H., Babu Rao, R. Indian J. Chem. 2001, 40B, 1270.
- Peterson, W. C. U.S. Pat. 4683000 Chem. Abstr., 1987, 107, 217644a.
- Baasnes, B., Schwamborn, M., Santel, H., Joachim, L. K. Eur. Pat. Appl. Ep. 422470, Chem. Abstr. 1991.
- Hiremath, S. P., Ullagaddi, A., Purohit, M. G. Indian J. Chem. 1988, 27B, 1102.
- Ali, R.; Mishra, B.Nizamuddin. Indian J. Chem. 1989, 28B, 526.
- Popp, F.D. Advances in Heterocyclic Chemistry 1975, 18, 1.
- Periyasami, G. Raghunathan, R. Surendiran, G. Mathivanan, N. Synthesis of novel spiropyrrolizidines as potent antimicrobial agents for human and plant pathogens, Bioorg. Med. Chem. Lett. 2008, 18, 2342-23425. (b) Kumar, R.S. Rajesh S.M., Perumal, S. Banerjee, D. Yogeeswari, P. Sriram, D. Novel three-component domino reactions of ketones, isatin and amino acids: Synthesis and discovery of antimycobacterial activity of highly functionalised amino acids: Synthesis and discovery of antimycobacterial activity of highly functionalised novel dispiropyrrolidines, Eur. J. Med. Chem. 2010, 45, 411-422.
- Stanley R., Jan B., Birgitta S. Ear. J. Org. Chem.2004, 4,13.
- Abdel-Aziz, S., El-Ahl, Heteroatom Chem. 2002, 13, 324.
- Padwa, A., Trost B. M and Fleming, I. Eds., ComprehensiveOrganic Synthesis, vol. 4, Pergamon,Oxford, UK, 1991.
- Arun, Y Bhaskar, G. Balachandran, C. Ignacimuthu, S. Perumal, P.T. Bioorg.Med. Chem. Lett. 2013, 23, 1839-1845.
- Bhaskar , G. Arun, Y. Balachandran, C. Saikumar, C. Perumal, P.T. Eur. J. Med.Chem. 2012, 51, 79-91.
- Fiswick, C. W. G. Foster, R. J. and Carr, R. E. “A short dipolarcycloaddition pproach to y-lactam alkaloids from cynometrahankei,” Tetrahedron Letters, 1996, 37, (22), 3915–3918.
- Jossang, A., Jossang, P., Hadi, H. A., Sevenet, T., Bodo, B. J. Org. Chem. 1991, 56, 6527.
- Lakshmi, N.V. Thirumurugan, P. Perumal, P.T. Tetrahedron Lett. 2010, 51, 1064-1068.
- Meyers, C., Carreira, E. M. J. Angew. Chem. Int. Ed. 2003, 115, 718.
- Moghaddam, F. M.; Boinee, H.Z. Tetrahedron 2004, 60, 6085.
- Molvi, K.I.; Mansuri, M.; Sudarsanam, V.; Patel, M.M.; Andrrabi, S. M. A.; Haque, N.J. En-zymeInhib. Med. Chem. 2008 23,829.
- Parai, M.K.; Panda, G.; Chaturvedi, V.; Manju, Y.K.; Sinha, S. Bioorg.Med.Chem.Lett. 2008, 18, 289.
- Wardakhan, W.; Abdel-Salam, O.; Elmegeed,G. Acta Pharm. 2008, 58,1.
- Sharma, S.; Athar, F.; Maurya, M. R.; Azam, A.Eur. J. Med. Chem. 2005, 40, 1414.
- Thangamani, A. Eur. J. Med. Chem. 2010, 45, 6120.
- Murray, P.R., E.J. Baroon, M.A. Pfaller, F.C. Tenover and R.H. Yolke, Manual of Clinical Microbiology. 6th Edn., American Society for Microbiology, Washington, DC,1995.
- The crystal structure has been deposited at the Cambridge Crystallographic Data centre CCDC number: 1027334, molecular formula: C36H30N2O3S, unit cell parameters: a 35.9142(14), b 8.9797(4), c 22.3056(10) space group C2/c. Data acquisition: The Cambridge Crystallographic Data Center;deposit@ccdc.cam.ac.uk, http://www.ccdc.cam.ac.uk/deposit.
- Sheldrick, G.M. SHELXS-97 Program for the solution of Crystal Structures, University of Gottingen, Germany, 1997.
- Spek,A.L. PLATON, in: A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, 1999.

This work is licensed under a Creative Commons Attribution 4.0 International License.









