Short time Modification of Carboxylated Multi-wall Nanotubes with Amino uracil derivative
Shahab Zomorod bakhsh1*, Behrooz Mirza2, Shima Yazdizadeh1, Ehsan Tavahodi1
1Department of Chemistry, Mahshahr Branch, Islamic Azad University, Mahshahr, Iran.
2Department of Chemistry, South Tehran Branch, Islamic Azad University, Tehran, Iran.
DOI : http://dx.doi.org/10.13005/ojc/300358
Article Received on :
Article Accepted on :
Article Published : 01 Sep 2014
In this study, the chemical functionalization of carboxylated multi-walled carbon nano tubes (MWNT-COOH) by Amino uracil derivative via microwave- assisted amidation method have been investigated.The functionalized MWNTs were characterized by Fourier Transform Infrared spectroscopy (FT-IR), Raman spectroscopy, elemental analysis and scanning electron microscopy (SEM). The major advantage of this procedure is reducing the reaction time to the order of minutes and the amidation was completed in one step as compared to two in the conventional approach. The acid chloride formation step was eliminated here.
KEYWORDS:Functionalization; Multi wall nanotubes; Amino uracil
Download this article as:| Copy the following to cite this article: Bakhsh S. Z, Mirza B, Yazdizadeh S, Tavahodi E. Short time Modification of Carboxylated Multi-wall Nanotubes with Amino uracil derivative. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Bakhsh S. Z, Mirza B, Yazdizadeh S, Tavahodi E. Short time Modification of Carboxylated Multi-wall Nanotubes with Amino uracil derivative. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4499 |
Introduction
Amino uracil derivatives have been reported to possess diverse biological activities such as antibacterial, antihypertensive, antiviral, antitumor effects and integral backbones of several calcium channel blockers1 . Moreover, several alkaloids containing the dihydropyrimidine core unit have been isolated from marine sources, which also exhibit interesting biological properties 2. Most notably among these are the batzelladine alkaloids, which were found to be potent HIV gp-120-CD4 in hibitors3. Thus, syntheses of these heterocyclic compounds are of much current importance. Carbon nanotubes have been attracting increasing attention from chemists and scientists owing to their electronic, mechanical, optical, and chemical characteristics 4–7. Biomedical applications for MWNTs are being investigated actively because of their useful combination of size and physicochemical properties 8–10. CNTs are usually functionalized to generate defects on the sidewalls and can provide sites for the coordination chemistry. functionalization via conventional methods require additional days of reaction time. So, regarding the time consuming process of carbon nanotube functionalization via conventional methods, we decided to use microwave radiation for modification of MWNT-COOH.Functionalization under microwave radiation is known to be somewhat different, faster and more efficient than conventional chemical method 11-12 .In this paper, we are mainly concerned with the microwave- assisted chemical functionalization of MWNTs containing amine groups by modifying the MWNTs with derivatives of Amino uracil and by this functionalization, MWNT solubility increased and active sites for further reactions are provided.
Experimental
All reagents and solvents were obtained from Merck Chemical Inc and MWCNT–COOH (95% purity, 20–30 nm; Netvino Co. Ltd) were purchased and used as received. The FT-IR spectra were recorded using KBr tablets on a Nexus 870 FT-IR spectrometer (Thermo Nicolet, Madison, WI). FT-Raman spectra were recorded on 960 ES, 1064 Thermo Nicolet. SEM measurement was carried out on the XL30 electron microscope (Philips, Amsterdam, Netherlands) for study the morphology of the MWCNTs. Elemental analyses of carbon, hydrogen, nitrogen and sulphur were performed using a Series ΙΙ 2400(Perkin Elmer, Waltham, MA) .
Preparation derivatives of Amino uracil
We have found that the microwave assisted of reaction urea (1 mmol) with cyano acetic acid (1 mmol) under solvent-free conditions results in rapid formation of the corresponding Amino uracil (Figure1).The products were easily obtained by addition of water to the reaction mixture and recrystalization of crude products from ethanol.13
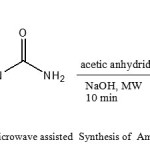 |
Fig1: Microwave assisted Synthesis of Amino uracil Click here to View Figure |
6-amino uracil, was obtained in 78% yield, as white powder, m.p>200 °C. IR: 3420, 3345 (N-H), 1660 (C=O), 1619, 1528 (C=C),
Preparation of MWNT- Amino uracil derivative
50 mg of the MWNT-COOH were mixed with 150 mg Amino uracil derivative and then sonicated in 20 ml of DMF for 30 minutes. Subsequently, the mixture was loaded into an extraction vessel and exposed to 300 W microwave irradiation for 30 min in several step mode. Then the reaction mixture was allowed to cool down and the mixture was filtered and washed thoroughly with ethyl alcohol and THF. Thus, the black solid was vacuum dried at room temperature for 6 hours.Corresponding chemical reaction is illustrated by Figure 2.
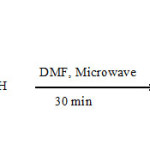 |
Figure 2 Click here to View Figure |
Results and Discussion
Raman spectra offer useful information concerning the slightly structural changes of MWNTs, especially the changes owing to significant sidewall modification. As can be seen in Figure 3, the characteristic peaks of MWNT tangential modes, namely the D band at around 1339 cm−1 and the G band at around 1596 cm−1 slightly changed. The frequencies of products are lower than that of MWNT-COOH, owing to the conjugated effect of uracil derivative 14-16. We observed an increase in the ratio of intensities ID/IG, from 0.68 to 1.15 in MWNT- Uracil derivative. This indicates an increased disorder of the graphitic structure of the modified nanotubes, which shows that the nanotubes were covalently modified.
 |
Fig3. Raman spectroscopy of A; carboxylated multi-walled carbon nanotube (MWNT-COOH) and B; modified multi-walled carbon nanotube ( MWNT- Amino uracil derivative)Click here to View Figure |
Fig.4 shows the FT-IR spectrum of the modified MWNTs. In spectrum A, the band at around 1527 cm-1 corresponds to the stretching mode of the C =C double bond that forms the framework of the carbon nano tube Sidewall 17. The peaks at 1704, 3304 cm-1 and 1071 cm-1 apparently corresponds to the stretching modes of the carboxylic acid groups. In spectrum B The carbonyl peak shift to 1646-1695 cm-1 (as compared with 1704 cm-1 in spectrum A) is a result of amid (C =O)NH linkage formation and the peaks at 3304 (OH) is removed.
 |
Fig4: Fourier transform spectra of A; carboxylated multi-walled carbon nanotube (MWNT-COOH) and B; modified multi-walled carbon nanotube ( MWNT- Amino uracil derivative) Click here to View Figure |
More direct evidence for the functionalization of MWNTs comes from the SEM images. Figure5 indicates that the MWNT–COOH has a smooth surface. The changes in morphology are remarkable. It seems that the diameters of MWNT– Amino uracil derivative are slightly increased in comparison with MWNT–COOH. The structure in MWNT– Amino uracil derivative is quite different from those of the starting MWNT–COOH, in which the tube surface is relatively smooth and clean, as shown in Figure 5.18
 |
Fig5: Scanning electron microscopy images of A carboxylated multiwalled carbon nanotubes and B MWCNT- Amino uracil derivative. Click here to View Figure |
Elemental analyses of the MWNT-COOH (A) and MWNT- Amino uracil derivative (B) are shown in Table 1. Apart from the carbon values, the atomic percentages of hydrogen, nitrogen and sulphur of B (as compared with A) indicated that A is functionalized with Amino uracil derivative.
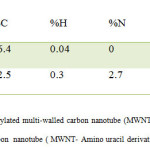 |
Table1: Elemental analysis of A; carboxylated multi-walled carbon nanotube (MWNT-COOH) and B; modified multiwalled carbon nanotube ( MWNT- Amino uracil derivative) Click here to View table |
A dispersion test gives a fair idea whether the modification on the carbon nanotubes has been achieved or not. Figure 6 presents a photograph of two vials containing MWNTCOOH and MWNT- Amino uracil dispersed in deionized water. As can be seen from Figure 6, MWNT-COOH are insoluble in deionized water while the modified CNTs can be directly dispersedin deionized water (without sonication) homogeneously and no precipitation was found even after it was sealed for 1 month at room temperature.
 |
Figure6: The images of MWNT-COOH and MWNT- Amino uracil in deionized water (1mg/6ml) after standing for 1 month. |
Conclusions
Many potential applications of carbon nanotubes often need them to be prior purified and functionalized. We have introduced Amino uracil derivative derivatives onto the surface of nanotubes under microwave radiation. The functionalized MWNTs was confirmed by Raman spectroscopy, FT-IR spectroscopy, elemental analysis and SEM images. The results show successful functional groups and by this functionalization, MWNT solubility increased and active sites for further reactions are provided.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Islamic Azad University Mahshahr Branch.
References
- B.B. Snider,J. Chen,A.D. Patil,A. Freyer, Tetrahedron Lett. 1996, 37, 6977–6980.
- L.E. Overman,M.H. Rabinowitz,P.A. Renhowe, J. Am. Chem. Soc. 1995 , 117, 2657.
- A.D.Patil,N.V.Kumar,W.C.Kokke,M.F.Bean,A.J.Freyer,C.DeBrosse,S.Mai,A.Truneh,D.J.Faulkner,J.Org.Chem. 1995, 60, 1182.
- Dresselhaus, M. S.; Dresselhaus, G.; Eklund, P. C. Science of Fullerenes and Carbon Nanotubes Academic, New York. 1996.
- Ebbesen, T. W. Carbon Nanotubes, 1997,35.
- Saito, R.; Dresselhaus, G.; Dresselhaus, M. S. Physical Properties of Carbon Nanotubes Imperial College Press. 1998.
- Iijima S. Helical microtubules of graphitic carb on. Nature. 1991, 354, 56–58.
- Bachtold A, Fuhrer MS, Plyasunov S. Phys Rev Lett. 2000, 84, 6082–6085.
- McEuen PL, Fuhrer MS, Park H. Single-walled carbon nanotube electronics. IEEE Trans Nanotech. 2002, 1, 78–185.
- Durkop T, Getty SA, Cobas E, Fuhrer MS. Nano Lett. 2004, 4, 35–39.
- Della Negra, F., Meneghetti, M., and Menna, E. Fullerenes, Nanotubes, Carbon Nanostruct., 2003, 11, 25–34.
- Wang, Y., Iqbal, Z., and Mitra, S. Carbon,2005, 43, 1015–1020.
- Devi, I.; Bhuyan, J. Tetrahedron Lett. 2005, 46, 5727.
- Banerjee, S., Hemraj-Benny, T., and Wong, S. S. Covalent surface chemistry of singlewalled carbon nanotubes. Adv. Mater.2005, 17, 17–29.
- Mitchell, C. A., Bahr, J. L., Arepalli, S., Tour, J. M., and Krishnamoorti, R. Macromolecules, 2002, 35, 8825–8830.
- Wu, H. L., Yang, Y. T., Ma, C. C. M., and Kuan, H. C. nanocomposites. J. Polym. Sci. Part A: Polym. Chem.,2005, 43, 6084–6094.
- Hu C Y, Xu Y J, Duo S W, Zhang R F, Li M S, J Chin Chem Soc., 2009, 56, 234.
- Bahr, J. L.; Yang, J.; Kosynkin, D. V.; Bronikowski, M. J.; Smalley, R. E.; Tour, J. M. J. Am. Chem. Soc. 2001, 123, 653 -6542.

This work is licensed under a Creative Commons Attribution 4.0 International License.









