Novel Synthesis and Anti-Microbial Activity Study of Innovative Mannich Bases Containing 2- Phenoxy-1, 3, 2-Dioxa Phospholanes And Indole Systems
Subba Narayana Kanchana*, Venkataramudu Burra, L. K. Ravindra Nath
Department in Chemistry, S.K. University, Anantapur, Andhra Pradesh, India
DOI : http://dx.doi.org/10.13005/ojc/300354
Article Received on :
Article Accepted on :
Article Published : 25 Sep 2014
In the present study, synthesis, characterization, antimicrobial activity on some novel pyrazolone derivatives has been taken up. The primary amines were diazotized with sodium nitrite and HCl mixture at 0-5 0C which were further coupled with ethyl acetoacetic ester to afford phenyl diazonium acetoacetic esters (II). Condensation of 4-substituted aryl hydrazono acetoacetic ester, 4-hydrazinylbenzenamine in the presence of catalytic amount of DMF under microwave irradiation afforded (4Z)-4-(2-substituted aryl hydrazono)-1-(4-aminophenyl)-3-methyl-1H-pyrazol-5(4H)-one (III). Subjecting (III) to anhydrous K2CO3 in DMF for 8 hours afforded 2-{4-[(4Z)-4-(2-substituted aryl hydrazono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenyl amino} acetate( IV) which was further reacted with hydrazine hydrate to afford 2-{4-[(4Z)-4-(2-substituted aryl hydrazono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenylamino} acetohydrazide (V). Reaction of (V) and Isatin (VI b) in DMF afforded (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5 oxopyrazol-1-yl)phenylamino)-N'-(2-oxoindolin-3-ylidene)acetohydrazide (VII). Compound (VII) was subsequently subjected to Mannich reaction with cyclic secondary amines (piperidine/morpholine/N-methyl piperidine) in the presence of formaldehyde in DMF to afford (4Z)-2-(4-((15Z)-4-(2-substituted aryl hydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N'-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene)acetohydrazide (VIII) which was characterized by I.R, 1HNMR and Mass data. The anti-bacterial activity of synthesized compounds was studied by the disc diffusion method against pathogenic bacteria and fungi.
KEYWORDS:Mannich Reaction; Mannich Base; Anti-microbial Activity
Download this article as:| Copy the following to cite this article: Kanchana S. N, Burra V, Nath L. K. R. Novel Synthesis and Anti-Microbial Activity Study of Innovative Mannich Bases Containing 2- Phenoxy-1, 3, 2-Dioxa Phospholanes And Indole Systems. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Kanchana S. N, Burra V, Nath L. K. R. Novel Synthesis and Anti-Microbial Activity Study of Innovative Mannich Bases Containing 2- Phenoxy-1, 3, 2-Dioxa Phospholanes And Indole Systems. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=5105 |
INTRODUCTION
Mannich bases and their derivatives have many attractive therapeutic use and applications, in paint and polymer chemistry as hardeners, cross linkers, reaction accelerations 1-2 etc. However, the most important applications are in the field of pharmaceutical products (3-4). Studies on antineoplastic drugs, analgesic drug, antibiotic drugs etc (5-9), including labeled molecules (10-12) have received particular attention in the recent past. Mannich bases can either directly be employed or used as intermediates in chemicals synthesis.
Literature review
All previous reported synthetic routes were reviewed and synthesis and biological activity of various Mannich bases are reported. Other similar synthetic routes also reported with different derivatives and all were reviewed completely (13-22).
Pandeya S.N and Usha L(23) et.al., Mannich condensation on 3-sulfadiazinylsarion in the presence of formaldehyde and secondary furnished 3-aminomethyl analogs which were evaluated as antimicrobial agents.
Pandeya S.N and Sriram D(24) et. al., have reported the synthesis and anti-HIV activity of Mannich bases of isatin.
Lingaiah N(25) et. al.,have reported the synthesis and anti-inflammatory activity of some 1-aminomethyl-3-benzoylhydrazono-2-indolinones.
Renukadevi P(26) et.al., have reported the synthesis and antimicrobial activity of 1-substitutedaminomrthyl-3-(5-substituted-3-phenylindol-2-yl-carbohydrazide) -2-indolinones.
Pandeya et al(27)Bhat AR Shenoy G.G and have reported the synthesis, antibacterial and antiviral activities of isatin derivatives.
Sridhar S.K28 et.al., Isatins have been reacted with 3-(4-pyridyl)-4-amino-5-mercapto-4H-1,2,4-triazole to from Schiff bases and the N-Mannich bases of these compounds were synthesized by reacting them with formaldehyde and several secondary amines. The compounds have been evaluated for their antimicrobial activity. Figure-1 represents the all published methods as discussed above.
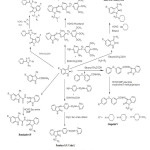 |
Figure1: Reported Mannich reactions Click here to View Figure |
Objective
Pyrazolones, pyrazoles and related hererocycles possess various types of biological activities. A good deal of importance is given to pyrazoline derivatives. It is due to their wide use in medical chemistry and some of them possess antituberculosis antineoplatic anti fertility and anti hydthyroid activity. The antibacterial activity of Mannich bases has been well established. In view of these observations, it appeared of interest to synthesize some novel Mannich bases bearing azomethine pyrazoline-5-one and indole moieties.
EXPERIMENTAL METHODS
Synthesis of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene) acetohydrazide Final product (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene) acetohydrazide involves eight steps. Each synthetic step reaction process and products information as discussed below.
Step-I: Synthesis of substituted diazonium chloride and Step-II: Synthesis of phenyl diazonium acetoacetic ester
The required substituted anilines were diazotized with sodium nitrite and HCl mixture at 0-50C and it is coupled with ethyl acetoacetic ester Step-I product to afford phenyl diazonium acetoacetic ester Step-II product.
Reaction process for step-I
The required primary amine is dissolved in a suitable volume of water containing 2.5-3.0 equivalents of hydrochloric acid (or sulfuric acid) by the application of heat of necessary. The solution thus obtained is cooled to 0ºC when the amine hydrochloride (or sulphate) usually crystallizes. The temperature is maintained at 0-5ºC, and the aqueous solution of sodium nitrite is added portion wise till there is free nitrous acid. The solution is tested for the later with an external indicator (moist potassium iodide starch paper). An excess of acid is always maintained to stabilize the diazoinum, acid is harmful; the concentration of the acid is reduced to optimum value. The similar procedure is adopted for the preparation of other substituted phenyl diazonium chlorides.
Reaction process for step-II
A solution of sodium acetate (1.0g) in 100ml of aqueous alcohol (50%) is added to a solution of ethyl acetoacetate (0.1 mol) in 50 ml of ethanol and the mixture is added to 0ºC. to this cold mixture, the corresponding diazonium chloride is added gradually till turbidity is observed. The addition is continued till yellow crystals separated out. These crystals are filtered, washed with water and dried.
Step-III: Synthesis of (4Z)-4-(2-substituted aryl hydrazono)-1-(4-aminophenyl)-3-methyl-1H-pyrazol-5(4H)-one
Condensation of 4-substituted aryl hydrazono acetoacetic ester, 4-hydrazinylbenzenamine in the presence of catalytic amount of dimethyl formamide under microwave irradiation afford (4Z)-4-(2-substituted aryl hydrazono)-1-(4-aminophenyl)-3-methyl-1H-pyrazol-5(4H)-one. In a typical experimental procedure, a mixture of aryl hydrazono acetoacetic ester, 4-hydrazinylbenzenamine and dimethyl formamide (10 drops) was subjected to microwave irradiation at 150 W intermittently at 30 sec intervals for 2 minutes.
After complete conversion as indicated by TLC, the reaction mixture was cooled and treated with cold water. The precipitate (4Z)-4-(2-substituted aryl hydrazono)-1-(4-aminophenyl)-3-methyl-1H-pyrazol-5(4H)-one was filtered recrystallized from ethanol. The yield is 88%
Reaction process
Condensation of 4-substituted aryl hydrazono acetoacetic ester step-II product and 4-hydrazinylbenzenamine in the presence of catalytic amount of dimethyl formamide under microwave irradiation afforded (4Z)-4-(2-substituted aryl hydrazono)-1-(4-aminophenyl)-3-methyl-1H-pyrazol-5(4H)-one. In typical experimental procedure, a mixture of arylhydrazono acetic ester step-II product, 4-hydrazinylbenzenamine and dimethyl formamide (10 drops) was subjected to microwave irradiation at 150W intermittently at 30 sec intervals for 2 minutes. After complete conversion as indicated by TLC, the reaction mixture was cooled and heated with cold water. The precipitate Step-III product was filtered recrystallized from ethanol M.P. 182ºC, yield 88%.
Step-IV: Synthesis of ethyl 2-{4-[(4Z)-4-(2-substituted aryl hydrazono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenyl amino} acetate
A mixture of, anhydrous K2CO3 and DMF was stirred at room temperature for 8 hours. The reaction mixture was diluted with ice cold water. The separated solid was identified as ethyl 2-{4-[(4Z)-4-(2-substituted aryl hydrazono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenyl amino} acetate.
Reaction process
A mixture of Step-III product, anhydrous K2CO3 and DMF was stirred at room temperature for 8 hours. The reaction mixture was diluted with ice cold water. The separated solid was identified as Step-IV product. This was collected by filtration, and recrystallized from ethanol. M.P. 204ºC, yield 78%. Elemental analysis found C: 58.22%, H: 5.62%, N: 19.55%, O: 16.75%. Calcd: C: 58.32%, H: 5.59%, N: 19.43%, O: 16.65%.
Step-V: Synthesis (4Z)-2 -(4-((15Z)-4 -(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-
oxopyrazol-1-yl) phenylamino)-N’-(2-oxoindolin-3-ylidene) acetohydrazide
A solution of step-IV product and hydrazine hydrate in ethanol was refluxed for 5 hours. The reaction mixture was cooled and poured on to ice cold water with stirring. The separated solid was filtered, washed with water and recrystallized from ethanol to afford 2-{4-[(4Z)-4-(2-substituted aryl hydrazono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenyl amino} acetohydrazide.
Reaction process
A solution of step-IV product and hydrazine hydrate in ethanol was refluxed for five hours. The reaction mixture was cooled and poured into ice cold water with stirring. The separated solid was filtered washed with water and recrystallized from ethanol to afford step-V product.
Step-VI: Synthesis of Isatin
The synthon, isatin was prepared by the procedure described by Marvel and Heins41.
Reaction process for step-VI (a)
In a one litre R.B flask 22g of chloral hydrate and 300ml of water placed. To this 325g of crystallized sodium sulphate, 12g of aniline (in 75ml of water), 12ml of conc.HCl and 27g of hydroxyl amine hydrochloride (in 25ml of water) were added. The flask was heated over a wire gauge for about 40-45 min. after one or two minutes of vigorous boiling, the reaction mixture was cooled and the separated solid was filtered and air-dried, yield 16gm, M.P. 175ºC. (lit35. M.P.175ºC).
Reaction process for step-VI (b)
Ina one litre R.B. flask 8 ml of conc. H2SO4 was placed and warmed at 50ºC, to this 18g of dry is o-nitro so acetanilide was added. The temperature was kept between 60-70ºC using external cooling. After the addition of the iso-nitroso compound, the reaction mixture was heated to 80ºC and kept at this temperature for about 10 minutes. Then the reaction mixture was cooled to room temperature and poured on to crushed ice. The precipitated isatin was filtered with suction, washed several times with cold water to remove the H2SO4 and then dried in the air, yield 12g M.P.190ºC (lit35.M.P.189-192ºC).
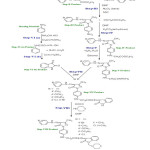 |
Figure2: Synthesis of (4Z)-2-(4-((15Z)-4-(2 phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene) acetohydrazide Click here to View Figure |
Step-VII: Synthesis of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5- oxopyrazol-1-yl)phenylamino)-N’-(2-oxoindolin-3-ylidene)acetohydrazide
Condensation of 2-{4-[(4Z)-4-(2-phenylhydrazono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenyl amino} acetohydrazide step-V product with Isatin step-VI product in DMF furnished the corresponding (4Z)-2-(4-((15Z)-4-(2-substituted aryl hydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxoindolin-3-ylidene)acetohydrazide step-VII product in excellent yields.
In a typical example, a mixture of step-V product (R=H) and step-VI product in 1:1 molar preparation when heated in DMF and water bath for 45 minutes yielded a compound M.P. 192ºC. Based on spectral data, the compound was assigned structure as (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxoindolin-3-ylidene)acetohydrazide Step-VII product (R=H).
Reaction process
A mixture of step-V product (R=H) and step-VI (b) product in 1:1 proportion heated in DMF (10 ml) on water bath for 45 minutes yielded a compound was filtered, washed with water and recrystallized from methanol to afford step-VII product yield M.P. 192ºC.
Step-VIII: Synthesis of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene) acetohydrazide
Compounds step-VII product was subjected to Mannich reaction with cyclic secondary amines (piperidine/morpholine/N-methyl piperidine) in the presence of formaldehyde in DMF to give (4Z)-2-(4-((15Z)-4-(2-substituted aryl hydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene)acetohydrazide Step-VIII product ( R = H (a)) in excellent yields.
In a typical example, a mixture of hydrazone step-VII product (R = H), with aqueous formaldehyde and piperidine in DMF for six hours at room temperature yielded a single product which was identified as (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3 –methyl -5 –oxopyrazol -1 -yl) phenylamino) -N’-(2-oxo-1-((piperidin-1-yl) methyl) indolin-3-ylidene)acetohydrazide step-VIII product ( R = H), on the basis of its spectroscopic data.
Reaction process
A mixture of hydra zone step-VII product (R=H) is stirred with aqueous formaldehyde and piperidine in DMF for 6 hours at room temperature and diluted with water. The solid thus separated was filtered, washed with water and recrystallized from methanol to give step-VIII product yield: M.P.:158ºC.
Similar synthesis
Similar synthesis has applied to other functional group derivatives and synthesized the other members of the series step-V to step-VIII products and their characterization also completed and characterization results are discussed in the results and discussion part. (R = CH3; OC2H5; Cl; H (b-f)).
RESULTS AND DISCUSSION
Step-V: Synthesis (4Z)-2 -(4-((15Z)-4 -(2-phenylhydrazono)-4,5-dihydro-3-methyl-5- oxopyrazol-1-yl) phenylamino)-N’-(2-oxoindolin-3-ylidene) acetohydrazide
The compounds synthesized Step-V Product have been characterized by means of their elemental analysis, I.R, 1NMR and Mass data.
IR Spectra
The IR (KBr) spectra of 2-{4-[(4Z)-4-(2-substituted aryl hydra zono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenyl amino} acetohydrazide Step-V Product (a) absorptions around 3445, 3425, (2 bands) 3305,1620,1665,1460 and 1455 cm-1 due to –NH2, >NH exo >C=N, cyclic carbonyl and five membered hetero cyclic ring respectively.
Table-1: IR spectral data for Step-V Product
|
Table-1: IR spectral data for Step-V Product |
||||||
|
Step-V Product |
R |
vmax |
||||
|
NH2 |
NH |
C = N |
C = O |
Mass (M) |
||
|
a |
H |
3445 |
||||
|
3425 |
3305 |
1620 |
1665 |
365.39 |
||
|
b |
CH3 |
3420 |
||||
|
3400 |
3285 |
1610 |
1650 |
379.42 |
||
|
c |
OCH3 |
3425 |
||||
|
3405 |
3200 |
1615 |
1555 |
395.42 |
||
|
d |
OC2H5 |
3435 |
||||
|
3415 |
3300 |
1615 |
1660 |
409.44 |
||
|
e |
Cl |
3420 |
||||
|
3400 |
3275 |
1610 |
1645 |
399.83 |
||
|
f |
Br |
3444 |
||||
|
3424 |
3290 |
1606 |
1650 |
444.29 |
||
1H NMR Spectrum
The 1H NMR (200MHz) spectra of 2-{4-[(4Z)-4-(2-phenylhydrazono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenyl amino} acetohydrazide Step-V Product a-f were recorded in DMSO-d6 proton 1H NMR spectrum of Step-V Product a is shown figure-2. The signal due to the methyl group appeared as a singlet at δ 1.0, integrating for three protons. The N-CH2-CO protons came into resonance at 3.85 as a singlet. The NH-N=C is appeared as singlet at 7.0
Table-2: 1H NMR spectral data for Step-V Product
|
Table-2: 1H NMR spectral data for Step-V Product |
||
|
Step-V Product |
R |
1HNMR (200MHz) (DMSO-d6) (δppm) |
|
a |
H |
1.2(s, 3H, CH3), 2.1(s, 2H, NH2), 3.8 (s, 2H, N-CH2-CO). 4.1 (s, 1H, Ar-NH), 6.8 (s, 1H, Ar-NH), 7.1- 7.3(m, 5H, C6H5) 7.4 (d, 2H, C6H4), 7.7 (d, 2H, C6H4), 8.35 (s, 1H, CONH). |
|
b |
CH3 |
0.9(s, 3H, CH3), 1.16 (s, 3H, CH3) 2.0 (s, 2H, NH2), 3.9(d, 2H, N-CH2-CO).4.0 (s,1H, Ar-NH), 6.9 (s, 1H, Ar-NH), 7.1- 7.3(m, 4H, C6H4) 7.4 (d, 2H, C6H4), 7.7 (d, 2H, C6H4), 8.35(s, 1H, CONH) |
|
c |
OCH3 |
1.1(s, 3H, CH3), 2.02 (s, 2H, NH2), 3.7 (s, 3H, OCH3), 3.95(d, 2H, N-CH2-CO),4.1(s,1H, Ar-NH), 6.9 (s, 1H, Ar-NH), 7.1- 7.3(m, 4H, C6H4), 7.4 (d, 2H, C6H4), 77 (d, 2H, C6H4), 8.35 (s,1H, CONH) |
|
d |
OC2H5 |
1.0(s, 3H, CH3), 1.1(t, 3H, CH3), 2.02 (s, 2H, NH2), 3.2 (q, 2H, O-CH2), 3.95(d, 2H, N-CH2-CO). 4.1 (s,1H, Ar-NH), 6.9 (s, 1H, Ar-NH), 7.1-7.3(m,4H, C6H4),7.4 (d, 2H, C6H4), 7.7 (d, 2H, C6H4), 8.35 (s,1H, CONH) |
|
e |
Cl |
1.1(s, 3H, CH3), 2.1 (s, 2H, NH2), 3.95(d, 2H, N-CH2-CO), 4.1 (s, 1H, Ar-NH), 6.8 (s, 1H, Ar-NH), 7.1-7.3 (m, 4H, C6H4), 7.4 (d, 2H, C6H4), 7.7(d, 2H, C6H4), 8.35 (s, 1H, CONH) |
|
f |
Br |
1.04(s, 3H, CH3), 2.0 (s, 2H, NH2), 3.9 (d, 2H, N-CH2-CO), 4.05 (s, 1H, Ar-NH), 6.8 (s, 1H, Ar-NH), 7.1-7.3 (m, 4H, C6H4), 7.4 (d, 2H, C6H4), 7.7(2H, C6H4), 8.35 (s, 1H, CONH) |
The NMR signal for CO-NH is noticed at δ8.4 as a broad singlet. The NH2 signal is observed at δ2.1 as a broad singlet.
Step-VII: Synthesis of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5- oxopyrazol-1-yl)phenylamino)-N’-(2-oxoindolin-3-ylidene)acetohydrazide
The structural assignments to these compounds Step-VII Product were based on the analytical and spectral data.
IR Spectra
The IR (KBr) spectra of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxoindolin-3-lidene)acetohydrazide Step-VII Product(a) showed characteristic strong absorption bands around 3205 (NH), 3170 (Indole NH), 1602 (C = N), 1656 (pyrazoline C = O), 1700 (C = O) and 1618 (CONH). The spectral data are recoded in Table – 3
Table-3: IR data for Step-VII Product
|
Table-3: IR data for Step-VII Product |
|||||||
|
Step-VII Product |
R |
vmax in cm-1 |
|||||
|
NH |
Indole NH |
C = N |
Pyrazoloine C = O |
Indole C = O |
CO–NH |
||
|
a |
H |
3205 |
3170 |
1602 |
1656 |
1700 |
1618 |
|
b |
CH3 |
3180 |
3140 |
1600 |
1654 |
1700 |
1622 |
|
c |
OCH3 |
3100 |
3150 |
1505 |
1654 |
1701 |
1625 |
|
d |
OC2H5 |
3195 |
3155 |
1604 |
1654 |
1701 |
1624 |
|
e |
Cl |
3175 |
3140 |
1605 |
1654 |
1701 |
1624 |
|
f |
Br |
3190 |
3150 |
1604 |
1654 |
1701 |
1624 |
1HNMR Spectra
The 1HNMR (200 MHz) spectrum of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxoindolin-3-ylidene)acetohydrazide Step-VII Product (a) in DMSO – d6 showed the following signals δ1.1 (s, 3H, CH3), δ10.93 (s, 1H, Ar-NH), δ5.78 (s, 2H, N-CH2-CO), δ12.75 (s, 1H, Indole NH), δ6.9-7.7 (m, 13H, Ar-H). The spectral data recorded in Table 4.
Table-4: 1HNMR spectral data for Step-VII Product
|
Table-4: 1HNMR spectral data for Step-VII Product |
||
|
Step-VII Product |
R |
1HNMR (200MHz) (DMSO-d6) (δppm) |
|
a |
H |
1.1 (s, 3H, CH3), 4.1 (s, 1H, Ar-NH), 5.8 (s, 2H, N-CH2-CO), 6.8 (s, 1H, Ar-NH), 7.0-7.1 (m, 9H, C6H4), 7.4 (d, 2H, C6H4), 7.7 (d, 2H,C6H4), 10.9 (s, 1H, CO-NH). 12.75 (s, 1H, Indole-NH) |
|
b |
CH3 |
1.0 (s, 3H, CH3), 1.15 (s, 3H, CH3), 4.15 (s, 1H, Ar-NH), 5.8 (N-CH2-CO), 6.8 (s, 1H, Ar-NH), 7.0-7.1(m, 8H, C6H4), 7.4 (d, 2H, C6H4), 7.7 (d, 2H, C6H4). 11.0 (s, 1H, CONH), 12.75 (s, 1H, Indole-NH). |
|
c |
OCH3 |
1.1 (s, 3H, CH3), 3.25 (s, 3H, OCH3), 4.1 (s, 1H, Ar-NH), 5.8 (N-CH2-CO), 6.8 (s, 1H, Ar-NH), 7.0-7.1(m, 8H, C6H4), 7.4 (d, 2H, C6H4), 7.7 (d, 2H, C6H4). 10.95 (s, 1H, CONH), 12.75 (s, 1H, Indole-NH). |
|
d |
OC2H5 |
1.0 (s, 3H, CH3), 1.2 (t, 3H, CH3), 3.2 (q, 2H, O-CH2) 4.15 (s, 1H, Ar-NH), 5.8 (N-CH2-CO), 6.8 (s, 1H, Ar-NH), 7.0-7.1(m, 8H, Ar-H), 7.4 (d, 2H, C6H4), 7.7 (d, 2H, C6H4). 10.9 (s, 1H, CONH), 12.75 (s, 1H, Indole-NH). |
|
e |
Cl |
1.05 (s, 3H, CH3), 4.1 (s, 1H, Ar-NH), 5.8 (s, 2H, N-CH2-CO), 6.8 (s, 1H, Ar-NH), 7.0-7.1 (m, 8H, C6H4), 7.4 (d, 2H, C6H4), 7.7 (d, 2H, C6H4), 10.9 (s, 1H, CONH), 12.75 (s, 1H, Indole-NH). |
|
f |
Br |
1.0 (s, 3H, CH3), 4.0(s, 1H, Ar-NH), 5.8 (s, 2H,N-CH2-CO),6.8 (s, 1H, Ar-NH), 7.0-7.1 (m, 8H, C6H4), 7.4 (d, 2H, C6H4), 7.7 (d, 2H, C6H4), 10.9 (s, 1H, CO-NH), 12.75 (s, 1H, Indole-NH). |
Mass spectra
The mass spectra of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxoindolin-3-ylidene)acetohydrazide Step-VII Product (a) (R=H) showed molecular ion (M+) peaks at m/z 494.
The mass spectral fragmentation patterns of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxoindolin-3-ylidene)acetohydrazide Step-VII Product (a) (R=H) are analyzed. The molecular ion observed at m/z 494 (15.5%), other important peaks appeared at m/z 477 (31.2%), 417 (16.5%), 389 (23.1%), 334 (100%) 323 (24.1%), 293 (12.1%), 277(21.9%), 202 (20.1%).
Step-VIII: Synthesis of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene) acetohydrazide.
The structure of this newly synthesized compounds VIII (a-f) where established on the basis of an elemental analysis and spectra data (IR and 1HNMR).
IR spectra
The IR (KBr) spectra of (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro -3-methyl -5 –oxopyrazol -1-yl) phenylamino)-N’-(2-oxo-1-((piperidin-1-yl) methyl) indolin-3-ylidene)acetohydrazide Step-VIII Product (a) (Mannich base) exhibited characterize bands around 3195 (NH), 1610 (C =N), 1676 (pyrazole C=O), 1654 (C – NH) and 2933cm-1 (CH2). The spectral data furnished in Table 5.
Table-5: IR Spectral data for Step-VIII Product
|
Table-5: IR Spectral data for Step-VIII Product |
||||||||
|
Step-VIII Product |
R |
X |
vmax in cm-1 |
|||||
| NH | C=N | Pyrazole C=O |
Indole C=O |
=O | NH | |||
|
a |
H |
CH2 |
3195 |
1610 |
1676 |
1720 |
1654 |
2933 |
|
e |
H |
O |
3193 |
1620 |
1681 |
1710 |
1660 |
2920 |
|
f |
H |
N |
CH3 |
3180 |
1617 |
1666 |
1710 |
1657 |
1HNMR Spectra
The 1HNMR (200 MHz) spectra of (4Z)-2-(4-((15Z)-4-(2-phenyl hydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl) methyl)indolin-3-ylidene) acetohydrazide Step-VIII Product (a) (R = H, X = CH2), 2-{4-[(4Z)-4 -(2-phenylhydrazono) -4, 5-dihydro -3 –methyl -5 –oxopyrazol -1 -yl] phenylamino}(2-oxo-1-morpholino-1-ylmethyl,2-dihydro-indol-3-ylidene)-acetohydrazide Step-VIII Product (g) (R =H, X = C) and (4E)-2-(4-((32Z)-4-(2-phenylhydrazono)-4,5-dihydro -3 –methyl -5-oxopyrazol-1-yl)phenylamino)-N’-(1-((4-methylpiperazin-1-yl) methyl)-2-oxoindolin-3-ylidene)acetohydrazide Step-VIII Product (h) (R = H, X = N-CH3) were recorded in DMSO-d6, the data are recoded in Table 6. The appearance of signal at δ4.5 due to N- CH2 – N, confirmed the formation of Mannich bases.
Table-6: 1HNMR Spectral data for Step-VIII Product
|
Table-6: 1HNMR Spectral data for Step-VIII Product |
|||
|
Step-VIII Product |
R |
X |
1HNMR (200MHz) (DMSO-d6) (δppm) |
|
a |
H |
CH2 |
1.1 (s, 3H, CH3), 1.45 [(s, 6H, (CH3)2], 2.56 (t, 4H,- CH2-N-CH2), 4.1 (s, 1H, Ar-NH), 4.5 (s, 2H, -N-CH2-N), 5.9 (s, 2H, N-CH2-CO), 6.8 (s, 1H, Ar-NH), 7.1-7.2 (m, 5H, C6H4), 7.4(d, 2H, C6H4), 7.7(d, 2H, C6H4), 9.0 (s, 1H, CONH). |
|
e |
H |
O |
1.1 (s, 3H, CH3), 2.6 (t, 4H, CH2-N-CH2), 3.7 (t, 4H, CH2-O-CH2), 4.15(s, 1H, Ar-NH), 4.5 (s, 2H, N-CH2-N), 6.0 (s, 2H, N-CH2-CO), 6.8 (s, 1H, Ar-NH), 7.1-7.2 (m, 5H, C6H4), 7.4(d, 2H, C6H4), 7.7 (d, 2H,C6H4), 9.0 (s, 1H, CONH). |
|
f |
H |
N-CH3 |
1.1 (s, 3H, CH3), 2.4 (t, 4H, CH2-N-CH2), 2.7 (t, 4H, CH2-N-CH2), 4.0 (s, 1H, Ar-NH),4.2(s, 3H, N-CH3), 4.5 (s, 2H, N-CH2-N), 6.0 (s, 2H, N-CH2-CO), 6.8 (s, 1H, Ar-NH), 7.1-7.2 (m, 5H, C6H4), 7.4(d, 2H, C6H4), 7.7 (d, 2H, C6H4), 9.0 (s, 1H, CONH). |
Mass spectra
The mass spectra of (4Z)-2-(4-((15Z)-4-(2-phenyl hydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene)acetohydrazide Step-VIII Product (a) (R= H, X = CH2) exhibited the molecular (M+) ion peak at m/z 591.
The fragmentation pattern analyzed with mass spectrum for (4Z)-2-(4-((15Z)-4-(2-phenyl hydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl)phenylamino)-N’-(2-oxo-1-((piperidin-1-yl)methyl)indolin-3-ylidene). The molecular ion (M+) “A” was observed at m/z 591 (12.5%), other important peaks appeared at m/z 562 (21.4%), 548 (25.8%), 472 (20.1%), 452 (17.2%), 420 (31.4%), 390 (14.5%), 334 (100%), 277 (28%).
Table-7: Characterization data for Step-V Product
|
Table-7: Characterization data for Step-V Product |
||||||||||
|
Step-V Product |
R |
M.P 0C |
Yield (%) |
Mol. Formula |
Found (%) – Calcd (%) |
|||||
|
C |
H |
N |
O |
Cl |
Br |
|||||
|
a |
H |
152 |
65 |
C18H19N7O2 |
59.25 |
5.32 |
26.90 |
8.83 |
||
|
(59.17) |
(5.24) |
(26.83) |
(8.76) |
|||||||
|
b |
CH3 |
153 |
60 |
C19H21N7O2 |
60.24 |
5.65 |
25.92 |
8.55 |
||
|
(60.15) |
(5.58) |
(25.84) |
(8.43) |
|||||||
|
c |
OCH3 |
156 |
75 |
C19H21N7O3 |
57.80 |
5.43 |
24.84 |
12.29 |
||
|
(57.71) |
(5.35) |
(24.80) |
(12.14) |
|||||||
|
d |
OC2H5 |
168 |
80 |
C20H23N7O3 |
58.79 |
5.78 |
24.09 |
11.84 |
||
|
(58.67) |
(5.66) |
(23.95) |
(11.72) |
|||||||
|
e |
Cl |
174 |
75 |
C18H18ClN7O2 |
54.22 |
4.70 |
24.67 |
8.24 |
9.01 |
|
|
(54.07) |
(4.54) |
(24.52) |
(8.00) |
(8.87) |
||||||
|
f |
Br |
169 |
65 |
C18H18BrN7O2 |
48.78 |
4.26 |
22.17 |
7.26 |
18.07 |
|
|
(48.66) |
(4.08) |
(22.07) |
(7.20) |
(17.98) |
||||||
Table-8: Characterization data for Step-VII Product
Table-8: Characterization data for Step-VII Product |
||||||||||
|
Step-VII Product |
R |
M.P 0C |
Yield (%) |
Mol. Formula |
Found (%) – Calcd (%) |
|||||
|
C |
H |
N |
O |
Cl |
Br |
|||||
|
a |
H |
214 |
70 |
C26H22N8O3 |
63.34 |
4.66 |
22.71 |
9.86 |
||
|
(63.15) |
(4.48) |
(22.66) |
(9.71) |
|||||||
|
b |
CH3 |
241 |
70 |
C27H24N8O3 |
63.95 |
4.94 |
22.17 |
9.61 |
||
|
(63.77) |
(4.76) |
(22.03) |
(9.44) |
|||||||
|
c |
OCH3 |
234 |
70 |
C27H24N8O4 |
62.00 |
4.81 |
21.51 |
12.34 |
||
|
(61.82) |
(4.61) |
(21.36) |
(12.20) |
|||||||
|
d |
OC2H5 |
224 |
75 |
C28H26N8O4 |
62.62 |
5.07 |
20.95 |
12.07 |
||
|
(62.44) |
(4.87) |
(20.81) |
(11.88) |
|||||||
|
e |
Cl |
225 |
75 |
C26H21ClN8O3 |
59.19 |
4.16 |
21.32 |
9.19 |
6.88 |
|
|
(59.04) |
(4.00) |
(21.18) |
(9.07) |
(6.70) |
||||||
|
f |
Br |
243 |
80 |
C26H21BrN8O3 |
54.64 |
3.83 |
19.70 |
8.49 |
8.55 |
|
|
(54.46) |
(3.69) |
(19.54) |
(8.37) |
(13.94) |
||||||
Table-9: Characterization data for Step-VIII Product
|
Table-9: Characterization data for Step-VIII Product |
|||||||||||
|
Step-VIII Product |
X |
R |
M.P 0C |
Yield (%) |
Mol. Formula |
Found (%) – Calcd (%) |
|||||
|
C |
H |
N |
O |
Cl |
Br |
||||||
|
a |
H |
CH2 |
158 |
70 |
C32H33N9O3 |
65.13 |
5.76 |
21.50 |
8.29 |
||
|
(64.96) |
(5.62) |
(21.31) |
(8.11) |
||||||||
|
(64.24) |
(5.87) |
(19.83) |
(10.07) |
||||||||
|
e |
Cl |
CH2 |
161 |
75 |
C32H32ClN9O3 |
61.54 |
5.30 |
20.22 |
7.78 |
5.82 |
|
|
(61.39) |
(5.15) |
(20.13) |
(7.67) |
(5.66) |
|||||||
|
f |
Br |
CH2 |
160 |
80 |
C32H32BrN9O3 |
57.45 |
4.95 |
18.93 |
7.28 |
12.03 |
|
|
(57.32) |
(4.81) |
(18.80) |
(7.16) |
(11.92) |
|||||||
|
(62.72) |
(5.26) |
(21.24) |
(10.78) |
||||||||
MICROBIAL ACTIVITY
Mannich bases step-VIII product (a,e,f) have good antifungal activity against Aspergillus Niger NCCS 1196 and Candida albicans NCCS 2106. In this series chloro, bromo and nitro, p-phenyl syndronyl, p-tolyl syndronyl and N-phenyl syndronyl showed good antifungal activity against Aspergillus Niger and Candida albicans at the concentration of 250 µg/ml.
ACKNOWLEDGEMENTS
The authors express their grateful thanks to Sri Krishnadevaraya University- Anantapur, for providing support for this research.
SUMMARY AND CONLUSION
Synthesis and biological activity of Mannich bases has developed and characterized. In this research, authors have developed mannich bases with new synthetic process.
Product-I: (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol- 1- yl) phenylamino)-N’-(2-oxoindolin-3-ylidene) acetohydrazide.
Product-II: (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5- oxopyrazol-1-yl) phenylamino)-N’-(2-oxoindolin-3-ylidene) acetohydrazide.
Product-III: (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol- 1-yl) phenylamino)-N’-(2-oxo-1-((piperidin-1-yl) methyl) indolin-3-ylidene) acetohydrazide.
A solution of ethyl 2-{4-[(4Z)-4-(2-substituted aryl hydrazono)-4, 5-dihydro-3-methyl-5-oxopyrazol-1-yl] phenyl amino} acetate Step-IV product and hydrazine hydrate in ethanol was refluxed for five hours afforded (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol- 1- yl) phenylamino)-N’-(2-oxoindolin-3-ylidene) acetohydrazide Step-V product Condensation of 4 with isatins Step-VI product afforded (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono) -4,5 –dihydro -3-methyl -5-oxopyrazol-1-yl) phenylamino)-N’-(2-oxoindolin-3-ylidene) acetohydrazide Step-VII product. Amino methylation of Step-VII product with formaldehyde and cyclic secondary amines (piperidine/morpholine/N-methyl piperazine) in DMF furnished (4Z)-2-(4-((15Z)-4-(2-phenylhydrazono)-4,5-dihydro-3-methyl-5-oxopyrazol-1-yl) phenylamino)-N’-(2-oxo-1-((piperidin-1-yl) methyl) indolin-3-ylidene) acetohydrazide Step-VIII product (Mannich bases).
The structures of these newly synthesized compounds Step-V product, Step-VII product and Step-VIII product were established on the basis of their elemental analysis and spectral (IR, 1HNMR and MS) data.
REFERENCES
- Tramontini, M.; Angioloni, L. Mannich-Bases, Chemistry and Uses, CRC, Boca Raton, F.L. (1994).
- Ared, M.; Westermann, B.; Risch, N. Angew. Chem Int Ed. 1998, 37, 1044.
- Sridhar, S. K.; Pandya, S. N.; Stables, J. P.; Ramesh, A. Eur. J. Pharm. Scien., 2002, 16(3), 129.
- Dimmock, J. R.; Kumar, P.; Curr. Med. Chem. 1977, 4(1), 1-22.
- Poplevskaya, I. A.; Kondauoru, G. N.; Abdullin, K. A.; Shipunova, L. K.; Chermanova G. B.; Kabiev, O. K. Tr. Inst. Khim. Nauk. Kaz, SSR. 1980, 52, 52; Chem. Abstr., 1981, 94, 120781.
- Against, P. C.; Krisch,G.; Wierzbicki, M.; Lepage, F.; Cagniant, D.; Loebenberg, D.; Parmergianin and Scherlock, R. Eur. J. Med. Chem. 1980, 15, 439.
- Korea Inst. Of Science and Technology. Jpn. Kokai Tokkyo Koho, JP. 1983, 5, 867, 693. Chem. Abstr. 1983, 99, 7047A.
- Dimmock, J. R.; Raghavan, S. K.; Logan, B. M.; Bigam, G. E. Eur. J. Med. Chem. 1983, 18, 249.
- Bundgard, H.; Methods in Enzymilogy. 1985, 112, 347.
- Masuda, K.; Toga, T.; Hayashi, N. J. Labelled Compd. 1975, 11, 301; Chem. Abstr. 1976, 84, 121730f.
- Fowler, J. S. J. Org. Chem. 1977, 42, 2637.
- Schreier, E. Helv. Chim. Acta. 1977, 59, 585.
- Gadre, J. N.; Dubhashi, V. B. Indian J. Heterocycl. Chem. 1994, 3, 181.
- Gahane, D. R.; Khapekan, K. D, Sharma, V. C.; Gaikwad, N. J. Indian J. Pharm. Sci, 1998, 60, 275.
- Rajasekaran, A.; Mahesh, R.; Parimoo P, Indian J.Heterocycl Chem. 1998, 8, 151.
- Balakrishna, K.; Prashantha, G.; Ananda, K. Indian J. Chem. 1999, 38B, 1295.
- Pandeya, S. N.; Shiram, D.; Nath, G.; Declercq, E.; Indian J. Pharm. Sci. 1999, 9, 358.
- Sridhar, S. K.; Pandeya, S. N.; Bajpai, S. K.; Manjula, H. Indian Drugs. 1999, 36, 412.
- Mojtahedi, M. M.; Sharifi, A.; Mohsenzadeh, F.; Saidi, M. R. Synth Commun. 2000, 30, 69.
- Baruah, S. G.; Prajapati, D.; Sandhu, J. S. Synlett., 2000, 15, 341.
- Pandeya, S. N.; Sriram, D.; Nath, G.; Declercq, E. Arznein-Forsch/Drug Res. 2000, 50, 55.
- Pandeya, S. N.; Sriram, D.; Nath, G.; Declercq, E. Eur. J. Med. Chem. 2000, 35, 249.
- Pandeya, S. N.; Usha, L. A.; Bajpal, S. K. Indian J. Heterocycl. Chem. 1977, 6, 313.
- Pendeya, S. N.; Sriram. D.; DeClecq, E.; Pannecouque, C.; Witvrouw, M. Indian J. Pharm. Sci. 1998, 60, 207.
- Lingaiah, N.; Narender. R.; Dattatrey, M. A. Indian J. Chem, 1998, 378, 1254.
- Renukadevi, P.; Biradan, J. S. Indian J.Heterocycl Chem. 1999, 9, 113.
- Bhat, A. R.; Shenoy, G. G.; Mohan, K. Indian J.Heterocycl Chem. 2000, 9, 319.
- Sridhar, S. K.; Ramesh. Aj, Biol. Pharm. Bull. 2001, 24, 1149.
- Varma, R. S.; Rastogi, N. Indian J.Heterocycl Chem. 2001, 11, 123.
- Varma, R. S.; Pachauri, G. Indian J.Heterocycl Chem. 2002, 12, 49.
- Varma, R. S.; Rastogi, N.; Singh. A. K. Indian J.Heterocycl Chem. 2003, 13, 131.
- Varma, R. S.; Rastogi, N.; Singh, A. P.; Kapil, A. Indian J.Heterocycl Chem., 2004, 13, 205.
- Mogilaiah, K.; Reddy, Ch. S.; Vidya, K. Indian J.Heterocycl Chem. 2004, 14, 145.
- Egawa, H.; Miyamoto, T.; Minamida, A.; Nishimura, Y.; Okada, H.; Uno, H.; Motosumota, T. J. Med. Chem. 1984, 27, 1543.
- Balin, G. B.; Tan, W. L. Aust. J. Chem. 1984, 37, 1065.
- Kuroda, T.; Suzuki, F.; Tamura, T.; Ohmoti, K.; Hosie, H. J. Med. Chem. 1992, 35, 1130.
- Chen, K.; Kuo, S.; Hsiech, M.; Anthoner, K. J. Med. Chem. 1997, 40, 3049.
- Ferrarini, M.; Clendio, M.; Calderone, U.; Lovella, G. Eur. J. Med. Chem., 1998, 33, 383.
- Varma, R. S.; Nobles, W. L. J.Pharm. Sci. 1975, 34, 881.
- Cavier, R.; Royer, R.; Rips, R.; Rene, L. Chim. Ther. 1969, 4, 21.
- Rajopadhye, M.; Popp. F. D. Indian J.Heterocycl Chem. 1984, 21, 289.
- Varma, R. S.; Pandey, R. K. Indian J. Chem.1982, 21B, 157.
- Mogilaiah, K.; Srinivas, K.; Rama Sudhakar, G. Indian J. Chem. 2004, 43B, 2014.
- Marvel, C. S.; Heris, G. S. Organic Synthesis, Coll. 1941, 1, 327.
- Hawes, E. M.; Wibberley, D. G. J. Chem. Soc (C). 1966, 5, 315.
- Lax, W. J. Park, Chem (2). 1901, 1, 63.
- Weixandt, W. J. Park, Chem. 1984, 49, 323.

This work is licensed under a Creative Commons Attribution 4.0 International License.









