Micellization of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS 1000): Thermodynamics and related Solute solvent interactions.
Hadiatul Ain Binti Hasanuddin1, Meor Mohd Affandi MMR1, 2, Mohamed Salama1, Minaketan Tripathy1,3*
1Laboratory of Fundamental Pharmaceutics, Faculty of Pharmacy, Universiti Teknologi MARA (UiTM), 42300 Bandar Puncak Alam, Selangor, Malaysia. 2DDH Core, Universiti Teknologi MARA (UiTM), 40450, Shah Alam, Selangor Darul Ehsan, Malaysia. 3Brain and Neuroscience Communities of Research, Universiti Teknologi MARA (UiTM), 40450 Shah Alam, Selangor Darul Ehsan, Malaysia.
DOI : http://dx.doi.org/10.13005/ojc/300323
Article Received on :
Article Accepted on :
Article Published : 17 Sep 2014
TPGS-1000 has been shown to have wide array of potentials in the field of pharmaceutical sciences. The present study involves the determination of the critical micelle concentration (CMC) of TPGS-1000 in water so to revalidate the value from literature. The conductometric technique has been used for the determination of the CMC value. The obtained value is well agreed with that of the literature. The temperature dependence of the CMC in case of TPGS-1000 has been observed to be negligible. Further the observed specific conductance values during conductometric measurements have been used to evaluate the corresponding values of molar conductance. The values of activation energy related to the different concentrations of TPGS-1000 have been estimated using the Arrhenius equation. The thermodynamic parameters for the micellization process of TPGS-1000 in water have also been calculated.
KEYWORDS:micellization; molar conductance; activation energy; and thermodynamics
Download this article as:| Copy the following to cite this article: Hasanuddin H. A. B. , MMR M. M. A, salama M, Tripathy M. Micellization of D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS 1000): Thermodynamics and related Solute solvent interactions. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Hasanuddin H. A. B. , MMR M. M. A, salama M, Tripathy M. Micellization of D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS 1000): Thermodynamics and related Solute solvent interactions. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4743 |
Introduction
TPGS-1000 (TPGS, d-α-tocopheryl polyethylene glycol 1000 succinate) is one type of novel non-ionic surfactant with an average molecular weight of 1513 has been used in several pharmaceutical applications in both liquid and solid dosage forms for increasing solubility and bioavailability after it’s by FDA as a safe pharmaceutics adjuvant and also have been utilized in countless TPGS-based drug delivery system (DDS)(1).
The lipophilic alkyl tail is derived from tocopherol succinate while its hydrophilic polar head portion is formed from polyethylene glycol making the surfactant structure bulky, hence with greater surface area. Vitamin E TPGS-1000 has hydrophile–lipophile balance (HLB) value of about 13.2(2).
It is believed to exhibit amphipathic properties and can form stable micelles in aqueous vehicles above its critical micelle concentration(CMC) at concentration as low as 0.02 wt% (3, 4, 5).
Owing to the versatile physico chemical properties TPGS-1000 has been broadly investigated for its emulsifying, dispersing, plasticizer, gelling, spreading, detergency and solubilizing effects on poorly water-soluble drugs(5) .Though there have been several reports on TPGS-1000, pertaining to its micellar behavior and application aspects, no reports regarding the thermodynamics and solution properties of TPGS-1000 and the subsequent micellization are available. Thermo physical investigations can provide information regarding the involved thermodynamic and the solute-solvent interaction(r). Conductometric analyses of the surfactant solutions have been widely utilized to study the thermodynamics of the systems (6,7,8,9) [references]. Hence we intend to determine the CMC by conductometric technique so to revalidate the value from literature and to elucidate the involved energetics and thermodynamics with further explanation of the solute solvent interactions.
Experimental part
Analytical Reagent grade TPGS-1000 was obtained from Sigma Aldrich,USA and been kept in a vacuum desiccator over anhydrous CaCl2 until required. The solutions were prepared freshly by mass using a Metler balance with a precision of ± 0.01mg in doubly distilled deionized and degassed water, and conversion of molality to molarity have been done by standard expression(10).
A conductivity meter with accuracy of ±0.5 % and a conductivity cell (EUTECH Instruments –CON700) has been used for the measurement of conductivity of each sample. The conductance cell was equipped with a water circulating jacket, and the temperature has been controlled within ±0.02K with a water thermostat (WiseCircu). The cell constant was usually 1.01cm -1 which has been calculated by repeated measurements of KCl solutions. All data has been corrected with specific conductivity of pure water at the experimental temperatures.
Different concentrations (0.002 to 0.1 M) of TPGS-1000 were prepared separately in double distilled water using 100 ml conical flask. The solutions were filtered and the specific conductivities were measured at four different temperatures of 298.15, 303.15, 308.15 and 313.15 K. For each sample six data points have been considered to calculate the average.
The values of specific conductance were plotted against the concentration of TPGS-1000 in weight fraction scale and the CMC values have been estimated graphically. The different thermodynamic parameters were further computed from the values of CMC at different temperature.
Results and discussion
The degree of micellization is greatly affected by the nature of the solvent and the temperature as the hydrophobic and hydrophilic interactions change with temperature (11 ). In this report, the CMC for the TPGS-1000 in water system were verified at different temperatures and concentrations of TPGS-1000. A clear understanding of the thermodynamic behavior of this amphiphiles can be further explained by the experimental data of the conductance measurements that have been recorded.
The temperature dependency of TPGS-1000 micellization is observed to be non-significant as in case of all the studied temperatures. From the conductivity data, the same concentration of 0.02 wt% is observed to be the CMC as the evident in the (Figure-1). However the conducting values increased with increase in temperature which is attributed to the thermal energy that enhanced the mobility of the solute in solvent hence increasing the values of conductivity. The CMC is determined from the flick point of the plot between conductivities and the surfactant concentration representing the y-axis and x-axis respectively (12).
![Figure 1 – Plots between specific conductivities against the different concentrations of TPGS-1000 at different temperatures in water. [298.15 K, 303.15 K, 308.15 K, 313.15 K].](http://www.orientjchem.org/wp-content/uploads/2014/09/Vol30_No3_Micel_Hadi_fig1-150x150.jpg) |
Fig1: Plots between specific conductivities against the different concentrations of TPGS-1000 at different temperatures in water. [298.15 K, 303.15 K, 308.15 K, 313.15 K]. Click here to View Figure |
The thermodynamic parameters during micellization, namely, Gibbs free energy (∆⁰Gm), enthalpy (∆H⁰m) and entropy (∆S⁰m)can be derived from the temperature dependence of the CMC. The free energy change of the micellization process (∆⁰Gm), is calculated using the equation in the form of (13)
∆⁰Gm = RT ln XCMC —————————————————(1)
The enthalpy ∆⁰Hm , is obtained by employing Van’t Hoff’s equation that is in the form of ∆H⁰m = – RT2 (d ln XCMC /dT)…………………………… .(2)
Further the enthapy of micellization is estimated from the slope of the plot of ln XCMC vs 1/T. The slope of which shall represent -∆H⁰m/R and entropy change, ∆⁰Sm from the well known relationship of Gibbs – Helmholtz equation,
∆G⁰m =∆H⁰m – T ∆S⁰m………………………………………………………….(3)
The availability of these parameters at a range of temperatures in the water can give valuable insight into the principles which direct the formation of micelles. As can be seen (Table-1), the ∆⁰Gm values for TPGS-1000 in the presence of water are negative, which suggest the facts that the process of micellization of TPGS-1000 in water is a favorable and spontaneous process. Further ∆⁰Gm values related to the process of micellization are found to increase with increase in the temperature of the system. This further explain the fact that the increment of temperature further assists the process of micellization in terms of spontaneity as the system become more favorable because of the added thermal energy.
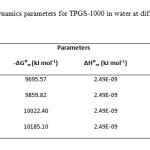 |
Table1: Thermodynamics parameters for TPGS-1000 in water at different temperatures.Click here to View table |
The positive ∆⁰Hm values for the systems containing TPGS-1000 show that the micellization processes are energy consuming or endothermic in nature while the positive ∆⁰Sm values for micellization indicated that the micellar species to be highly disordered in nature and entropy driven within the three-dimensional polymeric structure of water. These results further can be ascribed to the melting of “icebergs” or “flickering clusters” around the surfactant which can contribute to a non-random enhance in packing of hydrocarbon chains within the micellar core (14).
Since the mobility of an ion or a non ionic solute whether in solvated or non-solvated state is the important phenomenon for conductance measurement (15), it is quite reasonable to treat the conductance data similar to the one that used for the rate process taking place with the variation of temperature, i.e,
Λ= Ae-Ea/RT
Or
log Λ= log A-Ea/2.303RT…………………………………(4)
Where A is the frequency factor, R is the gas constant and Ea is the Arrhenius activation energy of the transport process in different concentration of TPGS-1000.From the plot of log Λagainst 1/T and the Ea value have been computed from the slope of (= -Ea/2.303R) are then recorded in (Table-2). From the result obtained, the calculated values of Ea are positive marked the fact that the process of micellization TPGS-1000 in water is favourable.
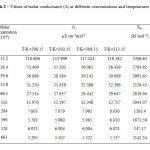 |
Table2: Values of molar conductance (Λ) at different concentrations and temperatures. Click here to View table |
The values of molar conductance (Λ)decrease with increase in concentration of the corresponding solution systems containing TPGS-1000 and increase with increase in temperature. These observations can explained by the fact that (i) the increase in TPGS-1000 concentration increases the micro viscosity of the system there by decreasing the mobility of the surfactant species, (ii) further at CMC the formation of micelles also hinders the mobility because of their enhanced hydrodynamic radii (iii) the increment in temperature causes an increased thermal energy which in turn results in greater bond breaking and variation in vibrational, rotational, and transitional energy of the solution systems containing TPGS-1000 that eventually lead to higher frequency and higher mobility(16).
Conclusion
In this study, the conductivities of TPGS 1000 in water at different concentrations are determined at four different temperature range between 298.15 and 313.15 K and it is found that the CMC values of TPGS 1000 are at 0.02 wt%. Besides that, based on the results of thermodynamics parameters, the negative values of the Gibbs free energy of micellization, ∆⁰Gm can lead to the conclusion that the formation of the micelles of TPGS-1000 in presence of water is a thermodynamically spontaneous process. The positive ∆⁰Hm values for the systems containing TPGS-1000 show that the micellization processes are energy consuming or endothermic in nature while the positive ∆⁰Sm values for micellization indicated that the micellar species to be highly disordered in nature and entropy driven within the three-dimensional polymeric structure of water. Moreover, the values of molar conductivities (Λ)decrease with increasing concentration of TPGS 1000 and increase with increase in temperature indicates that there is strong solute-solvent interaction in the system. In a nutshell, the positive values of activation energy, Ea of the system suggested an activation energy driven orientations of the surfactant leading to micellization in water.
References
- Wu, S.H.; Hopkins, W. K. Pharm tech. 1999, 23, 52-69 . .
- Ke, W.; Lin, S.; Ho, H.; Ming-Thau. J Cont Rel,2005, 102(2), 489-507.
- Sadoqi, M.; Lau-Cam, C. A.; Wu, S. H. J Colloid Interf Sci,2009 333(2), 585-589.
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B. O.; Zhang, Z. Euro J Pharm Sci, 2013 49(2), 175-186 (2013).
- Strickley, R. G. Pharm res, 2004, 21(2), 201-230.
- Mohamed M, Tripathy M, Majeed A.A, Arab J Chem, 2013, http :// dx. doi.. org/ 10.1016 /j . arabjc. 2013. 06. 022.
- Solanki C. S.; Tripathy S.; Tripathy M K.; Dash U N. E-J Chem, 2010, 7(S1), S223-230(2010).
- Tripathy S and Kar P. K, Orient J Chem, 2013, 29(3), 1103-1109.
- Tripathy S, Tripathy MK, Kar P K, Majeed A B A, Chem. Sci. Trans, 2013, 2(1), 208- 212.
- Robinson R. A and Stokes R. H, Electrolyte Solutions, Butterworth publications, London, 1955, 30.
- Sulthana, S.B.; Rao, PVC.; Bhat, SGT.; & Rakshit, A.K. J Physi Chem B,1998, 102(48), 9653-9660 .
- Ghosh K.K.; Baghel V. Ind J Chem, 2008, 47A,1230-1233.
- Tiwari L.K.; Mandal A.; Alam M.S.; Thennarasu S and Mandal A.B. Colloids Surf B: 2011, 82(1)126-133.
- Mosquera, V.; Ruso, J. M.; Attwood, David, J.; Malcolm N.; Prieto, G.; Sarmiento, F. J Colloid Interf Sci,1999, 210(1), 97-102.
- Coetzee J F and Ritchi D, Solute solvent interactions; Marcell Dekkar: New York and Basel, 1976.
- Dash, U.N.; Mahapatra, J.R.; & Lal, B. J Mol Liq,2006, 124(1), 13-18.

This work is licensed under a Creative Commons Attribution 4.0 International License.









