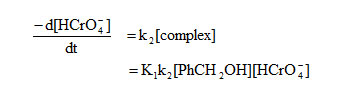Kinetics and mechanism of the selective oxidation of benzyl alcohols by acidified dichromate in aqueous acetic acid medium
K. Bijudas
Department of Chemistry, N. S. S. College, Manjeri, Malappuram, Kerala - 676122, India
DOI : http://dx.doi.org/10.13005/ojc/300360
Article Received on :
Article Accepted on :
Article Published : 01 Sep 2014
Kinetics of the oxidation of benzyl alcohol and some of its para substituted derivatives by acidified dichromate has been studied in acetic acid – water medium. The reaction showed first order dependence on both [substrate] and [oxidant]. The reaction failed to induce the polymerisation of added acrylonitrile and this rules out the involvement of any radical intermediate in the reaction. The reaction was found to be acid catalysed and showed fractional dependence on [acid]. The order of reactivity among the studied benzyl alcohols is p - OCH3 > p - CH3 > - H > p – Cl > p – NO2. Plots of log k2 versus Hammett's substituent constant (s) has been found to be curve shaped and this suggests that there should be a continuous change in transition state with changes in substituent present in the substrate from electron donating to electron withdrawing. Thermodynamic parameters were determined and a suitable mechanism is proposed in concordance with the obtained results.
KEYWORDS:Acidified dichromate; benzyl alcohol; kinetics; mechanism; selective oxidation; substituted benzyl alcohols
Download this article as:| Copy the following to cite this article: Bijudas K. Kinetics and mechanism of the selective oxidation of benzyl alcohols by acidified dichromate in aqueous acetic acid medium. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Bijudas K. Kinetics and mechanism of the selective oxidation of benzyl alcohols by acidified dichromate in aqueous acetic acid medium. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4481 |
Introduction
Hexavalent chromium in various forms has been widely used as mild and selective oxidizing agent in synthetic organic chemistry 1-2 and is mainly applied in the oxidation of primary and secondary alcohols to corresponding carbonyl compounds. There are many reports on such type of oxidations for synthesis of carbonyl compounds and on their kinetic aspects.3-7 But studies on structure – activity relationship are scanty and hence the present work is reported.
The present report deals with the kinetics of the oxidation of benzyl alcohol and substituted benzyl alcohols by acidified dichromate using acidified dichromate in aqueous acetic acid medium. Stoichiometry of the reaction, product analysis, effect of concentration of added acid, salt, substrate and oxidant, effect of polarity of the medium, effect of substituent and effect of temperature on the oxidation were carried out. Based on the results obtained activation parameters were computed. Structure – activity relationship was also studied by plotting the Hammett plot. A suitable mechanism is proposed based on the experimental observations.
Materials and methods
Analar grade potassium dichromate (Merck, India) was used and its solution was prepared in doubly distilled water. Analar Benzyl alcohol and acetic acid (Merck, India) were purified by reported methods.8 4 – methoxybenzyl alcohol, 4 – methylbenzyl alcohol, 4 – chlorobenzyl alcohol and 4 – nitrobenzyl alcohol (Lancaster, England) were used as such.
Kinetic measurements were carried out under conditions where [PhCH2OH] >> [K2Cr2O7]. The progress of the reaction was followed by titrating aliquots of the reaction mixture with standard sodium thiosulphate at regular intervals of time. The experiments were repeated and pseudo-first order rate constants, kobs were computed from the linear least square plots of log [oxidant] versus time.
Results and Discussion
Stoichiometry of the oxidation was established by equilibrating known excess concentration of potassium dichromate with known amount of benzyl alcohol. It was found that one mole of dichromate is equivalent to three moles of benzyl alcohol.
3PhCH2OH + Cr (VI) ® 3PhCHO + Cr (IV) + 6H+
3Cr (IV) ® Cr (VI) + 2Cr (III)
The product, corresponding benzaldehyde was identified as its 2,4 – dinitrophenylhydrazone (DNP) and is characterized by melting point and by various spectral techniques. The yield of the product was found to be above 85% and no traces of benzoic acid formed were detected. The oxidation of benzyl alcohol by potassium dichromate in an atmosphere of nitrogen failed to induce the polymerization of acrylonitrile and rules out the involvement of any radical intermediate in the reaction.
The effect of [H+] on the rate of oxidation of benzyl alcohol was studied by varying [H2SO4] under pseudo-first order condition and is given in table 1. The plot of log kobs versus log [H+] was linear with a positive slope and the order with respect to [H+] is found to be fractional. The linear increase in the oxidation rate with acidity suggests the involvement of a protonated Cr (VI) species in the rate-determining step.
![Table1: Effect of [H+] on the rate of oxidation.](http://www.orientjchem.org/wp-content/uploads/2014/09/Vol30_No3_Kine_Biju_T1-150x150.jpg) |
Table1: Effect of [H+] on the rate of oxidation.
|
The effect of added salt on the rate of oxidation was studied by varying the [NaCl] and is given in table 2. The rate constant is found to be almost constant with different concentrations of NaCl. This ruled an ion-ion type interaction and suggests the interaction between an ion and a dipole or between two dipolar entities.9-10
![Table 2: Effect of [NaCl] on the rate of oxidation.](http://www.orientjchem.org/wp-content/uploads/2014/09/Vol30_No3_Kine_Biju_T2-150x150.jpg) |
Table2: Effect of [NaCl] on the rate of oxidation.
|
The oxidation of benzyl alcohol was carried out with different initial concentrations of the oxidant and substrate in 20% aq. acetic acid medium with 1.0 x 10-2mol dm-3 H2SO4 at 308 K and rates were measured. The effect of [oxidant] and [substrate] on oxidation is given in table 3. The plot of log [oxidant] versus time was found to be linear at various [oxidant]. This proved that the reaction is first order with respect to [oxidant]. This was further confirmed from the constancy in the values of specific rates (kobs) for the different [oxidant] for a given concentration of the substrate. The effect [substrate] on the rate of oxidation was studied with different initial concentrations of the substrate and the observed rate constant increased linearly with the increase in [benzyl alcohol]. Further, the second order rate constants k2, were found to be constant indicating first order dependence of the reaction with respect to [substrate]. The first order dependence on [substrate] is further confirmed by the plot of log kobs versus log [substrate] which is linear with a slope of unity.
![Table 3: Effect of [oxidant] and [substrate] on the rate of oxidation of benzyl alcohol](http://www.orientjchem.org/wp-content/uploads/2014/09/Vol30_No3_Kine_Biju_T3-150x150.jpg) |
Table3: Effect of [oxidant] and [substrate] on the rate of oxidation of benzyl alcohol
|
The effect of substituents on the rate of oxidation of benzyl alcohol has been studied and is found in the order p – OCH3>p – CH3 > PhCH2OH > p – Cl > p – NO2. These results are presented in table 4. The electron-releasing substituents accelerate the oxidation process while the electron-withdrawing substituents retard the process.
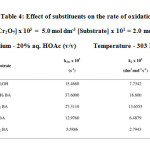 |
Table4: Effect of substituents on the rate of oxidation Click here to View table |
The effect of temperature on the rate of oxidation of benzyl alcohol and its derivatives using potassium dichromate in 20% aq. HOAc (v/v) in the presence of 1.0 x102 mol dm-3 H2SO4 were studied in the temperature range 303 K to 318 K. The values of various thermodynamic parameters were calculated and presented in Table 5.
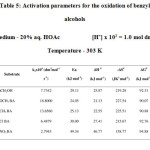 |
Table5: Activation parameters for the oxidation of benzyl alcohols |
The reaction was found to show first order kinetics with respect to [oxidant] and [substrate]. The effect of [H+] and [salt] on the rate of oxidation was studied. The effect of polarity of the medium on the rate of oxidation of benzyl alcohol was investigated by varying the acetic acid percentage in the reaction mixture. It has been observed that the reaction rate increases with increase in percentage of acetic acid (decrease in dielectric constant of the medium) suggesting more polar solvents may require larger reaction time. The plot of log k2 versus 1/D, where D is the dielectric constant of the medium is linear (r = 0.9978) with positive slope. This implies the occurrence of an interaction between a dipole and a positive ion and indicates the probable involvement of protonated Cr (VI) species in the reaction.
The reaction mixture failed to induce the polymerization of added acrylonitrile which rules out the involvement of any radical intermediate. This indicated that a one electron oxidation is not possible and supports the interaction between an ion and a dipole. The influence of substituents at the para position of the benzene ring of benzyl alcohol was studied and found that electron-releasing susbtituents accelerate the oxidation process while the electron-withdrawing groups retard the process. The plot of log k2 with s, where s is the susbtituent constant gave a smooth curve. The curved shape of Hammett’s plot may be due to gradual change in mechanism on passing from electron donating to electron withdrawing substituents or may be due to the change in rate determining step on changing substituents or due to change in the nature of transition state.
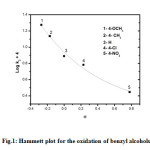 |
Fig1: Hammett plot for the oxidation of benzyl alcohols.
|
The activation enthalpies and entropies for the oxidation of benzyl alcohols are linearly related (r = 0.9969) as per Leffler Grunwald plot.12 The correlation between DH# and DS# was tested and found valid by applying Exner’s criterion13 by plotting log k2 at 303 K and log k2 at 313 K (r = 0.9946). The isokinetic temperature obtained from DH# Vs DS# plot is 361 K and that from Exner’s plot is 360 K. These results suggest that similar mechanism operates for the oxidation of all alcohols. This is further confirmed by the almost constant value of the free energy of activation (DG# » 92 kJmol-1) as given in Table 5.
Mechanism
It has been reported that the active species responsible for the oxidation of primary and secondary alcohols using Cr (VI) compound is HCrO4–.14-16 A hydrogen abstraction mechanism leading to the formation of free radicals is ruled out in view of the failure to induce the polymerization of acrylonitrile. The reaction is first order with respect to [oxidant] and [substrate]. The observations suggest the formation of an intermediate in a pre-equilibrium step and its subsequent disproportionation in the rate-determining step. It has been already established that oxidation of primary alcohols exhibits a substantial primary kinetic isotopic effect17-18. This confirmed the cleavage of a a C–H bond in the rate-determining step. The negative value of the polar reaction constant together with substantial deuterium isotope effect indicates that the transition state approaches a carbocation in character. Hence the transfer of hydride ion from the alcohol to the oxidant is suggested. The linear increase in the rate of oxidation with acidity suggests the involvement of protonated Cr (VI) species in the rate-determining step. The negative DS# values are in agreement with the formation of a chromate ester. Bordwell19has documented cogent evidence against the occurrence of concerted one step bimolecular process by hydrogen transfer. It is well established that intrinsically concerted sigmatropic reactions, characterised by transfer of hydrogen in a cyclic transition state are the only truly symmetrical processes involving a linear hydrogen transfer.20 Littler has also shown that a cyclic hydride transfer, in the oxidation of alcohols by Cr (VI), involves six electrons and being a Huckel type system, is an allowed process. 21 The overall mechanism is proposed to involve the formation of a chromate ester in a fast pre-equilibrium step and then a disproportionation of the ester in a subsequent slow step via a cyclic concerted symmetrical transition state leading to the product (Scheme 1).The observed hydrogen ion dependence can be explained by assuming a rapid reversible protonation of the chromate ester and the protonated ester decomposing at a faster rate than the chromate ester.
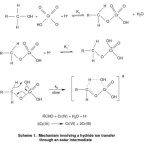 |
Scheme 1Click here to View Scheme |
A suitable rate expression for the oxidation of primary aromatic alcohols using potassium dichromate in consistent with the above mechanism can be given as
The oxidation of benzyl alcohols and para substituted benzyl alcohols by acidified dichromate in aqueous acetic acid medium gave corresponding benzaldehyde as the product. The oxidation was found to be highly selective since no traces of benzoic acid is detected. A suitable mechanism involving the formation of a chromate ester in a fast pre-equilibrium step and then the disproportionation of the ester in a subsequent slow step via a cyclic concerted symmetrical transition state forming the product was proposed.
References
- Lee D. G., Oxidation of organic compounds by permanganate ion and hexavalent chromium, Open Court: La Salle (1980)
- Wiberg K. B., Oxidation in organic chemistry, Academic Press, London and New York (1965)
- Joseph, J.; Basheer K. M.; Radhakrishnan Nair T. D. Asian J. Chem. 2007 19(6), 4733-4738
- Dave, I.; Sharma, V.; Banerji K. K. Indian J Chem. 2002, 41A, 493- 499
- Sharma, P. D.; Panchariya, P.; Purohit, P.; Sharma P. K. Eur. Chem. Bull. 2013, 2(10), 816- 824
- Basheer, K. M.; Joseph, J; Radhakrishnan Nair, T. D. Int. J. Chem. Sci. 2007, 5(3), 1191-1197
- Corey, E. J.; Suggs, J. W. Tetrahedron Lett. 1975, 31, 2647-2650
- Perrin D. D., Armarego W.L. and Perrin D.R., Purification of Organic Compounds, Oxford, Pergamon Press (1966)
- Bronsted, J. N. Z. Physik. Chem. 1925, 115, 337-364
- Bjerrum, B. Z. Physik. Chem . 1924,108, 182: 1925,118, 251
- Hammet L. P., Physical Organic Chemistry, 2nd Edn., Mcgraw Hill; Tokyo (1970)
- Leffler J. E. and Grunwald E., Rates and Equilibrium of Organic Reactions, Wiley; New York (1963)
- Exner, O. Collect Czech Chem Commun. 1964, 29, 1094-1101.
- Westheimer, F. H.; Novick A. J Chem Phys. 1943, 11, 506- 512
- Cohen, M.; Westheimer F. H. J Am Chem Soc. 1952.74, 4387-4391
- Kwart, H.; Francis, P. S. J Am Chem Soc. 1955, 77, 4907-4911
- Banerji, K. K. J. Chem. Soc. Perkin Trans 2. 1988, 547, 2065-2069.
- Banerji, K. K. J. Org. Chem 1988, 53, 2154-2159.
- Bordwell, F. G. Acc. Chem. Res. 1974, 5, 374-384.
- Woodward, R. B.; Hoffman, R. Angew. Chem. Int. Ed. Eng. 1969, 8, 781-932.
- Littler, J. S. Tetrahedron. 1971, 27, 81-91.

This work is licensed under a Creative Commons Attribution 4.0 International License.