Effect of gamma-irradiation on thermal decomposition of tris(1,2-diaminoethane)cobalt(II)sulphate
Jayashri T.A.1*, Krishnan G.2, Rema Rani N.1
1Department of Chemistry, University of Kerala, Thiruvananthapuram, 6 95581, Kerala, India
2Department of Chemistry, University College, Thiruvananthapuram, 695034, Kerala, India
DOI : http://dx.doi.org/10.13005/ojc/300345
Article Received on :
Article Accepted on :
Article Published : 24 Sep 2014
Tris(1,2-diaminoethane)cobalt(II)sulphate was prepared and characterised. Pure dry sample of uniform mesh size was then irradiated with 60Co ϒ-rays for varying doses up to 900 kGy. Non-isothermal decomposition of the complex was studied before and after irradiation. Unirradiated and irradiated samples decompose in three stages. Kinetic parameters E, Z and ∆S were evaluated by Coats-Redfern (integral), Freeman-Carroll (differential) and Horowitz-Metzger (approximation) equations. Results showed that irradiation enhanced thermal decomposition, lowering thermal as well as kinetic parameters. Mechanism of decomposition for each stage has been established. Irradiation modifies the reaction mechanism of the first stage of decomposition.
KEYWORDS:Tris(1,2-diaminoethane)cobalt(II)sulphate; ϒ-irradiation; Thermal decomposition kinetics; Mechanism
Download this article as:| Copy the following to cite this article: Jayashri T. A, Krishnan G, Rema Rani N. Effect of gamma-irradiation on thermal decomposition of tris(1,2-diaminoethane)cobalt(II)sulphate. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Jayashri T. A, Krishnan G, Rema Rani N. Effect of gamma-irradiation on thermal decomposition of tris(1,2-diaminoethane)cobalt(II)sulphate. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4991 |
INTRODUCTION
Pre-exposure to energetic radiation considerably affect the thermal decomposition kinetics of crystalline solids, the contributing factors being structural as well as topochemical. Inorganic salts containing oxyanions like acetates, chlorates, thiosulphates etc. have been extensively studied in this regard (1-3) while those of complexes are scanty (4, 5).In our earlier workwe have reported the effect of gamma irradiation on non-isothermal decomposition kinetics of bis(diethylenetriamine) cobalt(II)nitrate (6). In the present investigation the ligand is replaced by ethylenediamine and the counter ion nitrate, by sulphate. Thus the present paper reports the effect of gamma irradiation on non-isothermal decomposition kinetics of tris(1,2- diaminoethane)cobalt(II)sulphate. The study proposes evaluation of kinetic parameters by non-mechanistic equations for unirradiated and irradiated samples. The non-mechanistic equations employed are Coats-Redfern (integral), Freeman-Carroll (differential) and Horowitz-Metzger (approximation) (7-9). In order to establish the mechanism of thermal decomposition, evaluation of kinetic parameters by mechanistic equation is also intended.
EXPERIMENTAL
All chemicals used were of AR grade or of high purity. Hydrated cobalt sulphate and ethylenediamine were obtained commercially and used without further purification. Tris (1,2-diaminoethane)cobalt(II)sulphate was prepared by known procedure (10). The sample was purified by recrystallisation and dried over P2O5, characterized by elemental analysis, spectral and magnetic studies. The IR spectrum of the sample was recorded on a Perkin Elmer infrared spectrophotometer in the range 4000 to 500cm-1. Electronic spectrum was recorded on a Shimadzu uv-visible spectrophotometer in the range 300-900 n.m. at room temperature. Magnetic susceptibility was measured by Gouy method. Octahedral geometry of the complex ion was confirmed by electronic spectral and magnetic studies. Pure dry sample was ground and sieved to uniform mesh size and sealed in Pyrex ampoules.The sample was irradiated using 60Co gamma rays to varying doses as 300 kGy, 600 kGy and 900 kGy at constant intensity under room temperature at a dose rate of 6 kGy/hr. After irradiation each sample was mixed uniformly and stored over P2O5 in a vacuum desiccator. Thermograms providing simultaneously TG and DTG were recorded in a Perkin Elmer STA 6000 model instrument in nitrogen atmosphere at a heating rate of 10°C/min. The sample mass was kept constant around 3±0.5mg. Thermal studies were carried out within one week of irradiation.
RESULTS AND DISCUSSION
Thermal and kinetic analysis
The TG curves recorded in nitrogen atmosphere were redrawn as percentage mass versus temperature curves (Fig 1).
![Fig.1. Thermal decomposition curves redrawn as percentage of mass versus temperature curves for unirradiated and irradiated [Co (en)3]SO4](http://www.orientjchem.org/wp-content/uploads/2014/09/Vol30_No3_Effect_Jaya_fig1-150x150.jpg) |
Fig.1: Thermal decomposition curves redrawn as percentage of mass versus temperature curves for unirradiated and irradiated [Co (en)3]SO4 |
Thermograms of unirradiated and irradiated samples are of the same pattern indicating that irradiation does not modify the decomposition pathway. Both the unirradiated and irradiated samples (300 kGy, 600 kGy and 900 kGy) of the complex decompose in three stages. Percentage mass loss during the first stage corresponds to the removal of two mols of the ligand while that of the second stage to the remaining one mole. The third stage involves the oxidation of the deaminated complex to the metal oxide (Table 1).
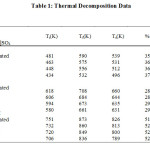 |
Table1: Thermal Decomposition Data Click here to View table |
The percentage of final residue in all cases corresponds to oxide of cobalt, which is confirmed by independent pyrolysis data. Phenomenological data with Ti– temperature of inception, Tf– temperature of completion and Ts– peak temperature for the unirradiated and irradiated samples are presented in Table 1. It can be seen that Ti, Tf, and Ts are lowered upon irradiation, indicating decomposition is relatively faster in the irradiated samples. Non-isothermal data were analyzed by Coats-Redfern, Freeman-Carroll and Horowitz-Metzgermethods (non-mechanistic equations). Kinetic parameters viz., activation energy E, frequency factor Z and entropy of activation ∆S along with the correlation coefficient (r) evaluated by these methods, are presented in Tables 2, 3 and 4.
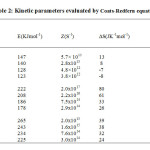 |
Table2: Kinetic parameters evaluated by Coats-Redfern equation Click here to View table |
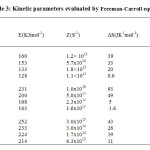 |
Table3: Kinetic parameters evaluated by Freeman-Carroll equation Click here to View table |
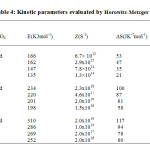 |
Table4: Kinetic parameters evaluated by Horowitz-Metzger equation Click here to View table |
Calculations were done by the statistical linear regression method using excel pre-defined functions. The order parameter n was assumed as unity. From Tables 1 – 4 it is clear that thermal as well as kinetic parameters are lowered upon irradiation and the extent of lowering increases with increase in absorbed dose. Thus the reactivity parameters follow the order, unirradiated > 300 kGy > 600 kGy > 900 kGy. The decomposition of the complex [Co (en)3] SO4 starts in the solid state. The decomposition reactivity of crystalline solids depends upon structural and energetic factors associated with the chemical nature of the reactants and products, lattice geometry and defect concentration. As irradiation leads to an increase in defect concentration and subsequent chemical damage, all these factors are modified. Thus the enhanced rate of decomposition may be attributed to lattice defects as well as products of chemical damage. These effects can influence both diffusion and nucleation which are the two fundamental steps in a solid state decomposition.
Deduction of mechanism
In the present investigation the mechanism is established by following the method of Sestak and Berggren (11) and Satava (12). In non-is othermal kinetic studies E is evaluated from the slope, tgβ of the straight line approximation of the plot of ln gα versus 1/T by using the equations proposed by Sestak (13). Computational details for arriving to the correct mechanism and the respective kinetic parameters have already been discussed (14). The kinetic parameters evaluated by mechanistic equations are presented in table 5.
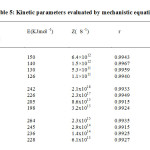 |
Table5: Kinetic parameters evaluated by mechanistic equation Click here to View table |
When the correlation coefficient is nearly the same the mechanism is chosen by comparing the E values with those obtained from the non-mechanistic equations. It is seen that in the first stage, the decomposition of unirradiated sample is governed by R3 function i.e. phase boundary reaction with spherical symmetry. In phase boundary reactions the reactant is assumed to have a definite geometrical form. The surface nucleation of the reactant occurs instantaneously and an interface will be established. This interface moves with a constant velocity towards the centre of the particle and the reaction is deceleratory throughout, because the reaction interface decreases continuously. Phase boundary reactions with spherical symmetry corresponds to three dimensional movement of the interface which is also known as contracting cube equation, i.e., [1- (1-α)1/3 ] = kt. The mechanism of decomposition changes to R2 function in the irradiated samples, i.e., phase boundary reaction with cylindrical symmetry. Phase boundary reaction with cylindrical symmetry corresponds to the two dimensional movement of the interface and follow the equation, [1- (1-α) 1/2 ] = kt. For the unirradiated and irradiated samples the second stage of decomposition is controlled by F1 function ie., random nucleation, one nucleus on each particle., -ln (1-α) = kt (Mampel equation).The third stage is governed by R3 function for both the unirradiated and irradiated samples.
CONCLUSION
In this work, non-isothermal decomposition kinetics of the complex tris(1,2-diaminoethane) cobalt(II)sulphate was investigated before and after gamma irradiation. It was found that irradiation enhanced thermal decomposition, lowering thermal as well as kinetic parameters, but did not change the decomposition path way. Irradiation modifies the reaction mechanism of the first stage of decomposition.
ACKNOWLEDGEMENTS
The authors are thankful to the authorities of Central University, Pondicherry, Department of Chemistry, University of Kerala and Govt. Arts College, Thiruvananthapuram for providing instrumental facilities. Rema Rani.N is thankful to the University of Kerala for the award of a Teacher Fellowship and U.G.C., Bangalore for financial assistance.
REFERENCES
- Mahfouz, R.M.; Al-Shehri, S.M.; Monshi, M.A.S.; Al-Haizan, A.I.; Abd El Salam, N.M.; Radiat.Eff. Def.Solids.2007, 163,95-100
- Nair, S.M.K.; Sahish, T.S.; J. Radioanal.Nucl.Chem.1993, 175,173-184
- Morsy Abou Sekkina.; El-Sayed El-Shereafy.; Mashaly A.; El-Ashry M.; J.Radioanal.Nucl.Chem. 1998,237,113-119
- Krishnan, G.; Jayashri, T.A.; Sudha, P.; Radiat.Phys.Chem. 2009,78,933-938
- Krishnan, G.; Jayashri, T.A.; Geetha Devi, K.; Radiat.Phys.Chem.2009, 78,184-190
- Jayashri, T.A.; Krishnan, G.; J.Radioanal.Nucl.Chem.2008, 3,693-697
- Coats, A.W.; Redfern, J.P.; Nature.1964, 201, 68-69
- Freeman, E.S.; Carroll, V.; J.Phys.Chem.1958, 62,394-397
- Horowitz, H.H.; Metzger, G.; Anal.Chem.1963, 35, 1464-1468
- Rochow, E.G (ed)., Inorganic Synthesis, Vol. VI.; McGraw-Hill. New York (1960)
- Sestak, J.; Berggren, G.; Thermochim.Acta.1971, 3, 1-12
- Satava, V.; Thermochim.Acta.1971, 2,423-428
- Sestak, J.; Thermochim.Acta.1971, 3,150-154
- Nair, S.M.K.; James, C.; Thermochim. Acta.1984, 78,357-370

This work is licensed under a Creative Commons Attribution 4.0 International License.









