Derived thermodynamic properties of binary mixtures of m-Xylene, o-Xylene, and p-Xylene, with N,N-Dimethylformamide at T = (293.15, 303.15, 313.15 and 323.15) K
Ezekiel Dixon Dikio*
Applied Chemistry and Nanoscience Laboratory, Department of Chemistry, Vaal University of Technology, P. O. Box X021, Vanderbijlpark1911, Republic of South Africa
DOI : http://dx.doi.org/10.13005/ojc/300306
Article Received on :
Article Accepted on :
Article Published : 29 Aug 2014
Experimental data on dynamic viscosities and densities of binary mixtures of N,N-dimethylformamide (DMF) and o-xylene, m-xylene and p-xylene were measured at 293.15, 303.15, 313.15, and 323.15 K. Computed data from experiment for deviations in viscosity, excess molar volume and excess Gibbs energy of activation of viscous flow were correlated with Redlish-Kister polynomial equation. Grundberg-Nissan interaction constant (d¢), Hind, Frenkel and a modified Kendall-Monroe equation (Ehm) were determined quantitatively. Deviation in viscosity were both negative and positive while excess molar volume and excess Gibbs energy of activation of viscous flow were all positive. These results have been interpreted on the basis of intermolecular interactions between unlike molecules.
KEYWORDS:Density; Viscosity; Thermodynamic parameters; Binary mixtures; Xylenes; N; N-dimethylformamide.
Download this article as:| Copy the following to cite this article: Dikio E. D. Derived thermodynamic properties of binary mixtures of m-Xylene, o-Xylene, and p-Xylene, with N,N-Dimethylformamide at T = (293.15, 303.15, 313.15 and 323.15) K. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Dikio E. D. Derived thermodynamic properties of binary mixtures of m-Xylene, o-Xylene, and p-Xylene, with N,N-Dimethylformamide at T = (293.15, 303.15, 313.15 and 323.15) K. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4443 |
Introduction
Intermolecular interactions have continued to capture the interest of chemists because of its important role in many chemical and biological systems.1Solvent systems are frequently used as media for many chemical, industrial and biological processes in that they provide a wide range of desired properties.2 Properties such as density and viscosity at several temperatures both for pure chemicals and their binary liquid mixtures over a whole composition range are useful for a full understanding of their thermodynamic and chemical engineering purposes.3Experimental data and excess thermodynamic properties of liquid mixtures are necessary in drawing information on the structure and interactions of liquid mixtures.4N,N-dimethylformamide (DMF) is a polar aprotic solvent with a high boiling point,5 a large dipole moment and high dielectric constant facilitate reactions that provide a good donor acceptor property.1-7In the pure state, N,N-dimethylformamide show association through dipole-dipole interaction.7It finds applications in polymer science and pharmaceutical industrial and serves as a model compound of peptides to obtain information on protein systems.6Formamides also find application in industrial processes that involve their use as synthetic intermediates in the preparation of fungicides and polymers such as polyacrylonitrile.8
Xylenes are frequently utilized as raw materials in many organic syntheses. High-purity xylenes are difficult to separate from common distillation operations, as it forms several binary azeotropes. N,N-dimethylformamide has been widely used as a selective extractant solvent for xylene separation from naphtha feed.1
Measurements of densities, viscosities, speeds of sound and other thermodynamic properties of binary mixtures ofo-, m-, p-xylene and isopropyl benzene and 2-butanone,9pyridine,101-decanol,11N,N-diethylformamide,8 acetic acid,12methylformate13have been studied.
There are apparently no studies in the literature of thermodynamic studies of densities, viscosities and excess properties of the xylenes with N,N-dimethylformamideat the temperatures 293.15, 303.15, 313.15 and 323.15 K.1,7
We have studied o-, m-, p-xylene with pyridine at 293.15, 303.15, 313.15 and 323.15 Kto determine inter- and intra-molecular interactions that exit in their binary mixtures10In this study, experimental viscosity and densities are reported at four temperatures 293.15, 303.15, 313.15 and 323.15 K for binary mixtures of m-xylene, o-xylene and p-xylene with N,N-dimethylformamide. Deviation in viscosity (Δη), excess molar volume (VE) and excess Gibbs free energy of activation of viscous flow (ΔG*E) have been calculated from the density (p), and viscosity (η), data. Modified Kendall-Monroe10,14,15 equation with no parameters has been used in correlating viscosity data of the binary mixtures. Calculated deviation in viscosity and excess functions were fitted to the Redlich-Kister16 polynomial equation and the results analyzed in terms of molecular interactions.
Experimental
Materials
Reagent grade m-xylene, o-xylene, p-xylene and N,N-dimethylformamidewere purchased from Sigma-Aldrich South Africa and used without further purification. The purity of the liquids was ascertained by comparing their measured densities (ρ), and viscosities (η), with those reported in the literature.8-17
Mixture preparation
Binary mixtures were prepared by weighing appropriate amounts of N,N-dimethylformamide and xylene on an electronic balance. An AE Adam balance (Adam Equipment Inc. USA) model PW124 with a maximum capacity of 120 g, a readability range 0.0001 g and repeatability (S.D.) of 0.00015 g, linearity 0.0002 g, operating temperature +10oC to 40oC was used in all measurements.
Density measurement
Density measurements were carried out with an Anton Paar DMA-4500 M digital densitometer thermostatted at different temperatures. Two integrated Pt 100 platinum thermometers were provided for good precision in temperature control internally (T ± 0.01 K). The densitometer protocol includes an automatic correction for the viscosity of the sample. The calibration for temperature and pressure was made by the producer. The apparatus is precise to within 1.0×10-5 g/cm3, and the uncertainty of the measurements was estimated to be better than ± 1.0×10-4 g/cm3. Calibration of the densitometer was performed at atmospheric pressure using doubly distilled and degassed water.
Viscosity measurement
Viscosity measurements were carried out using Anton Paar SVM 3000 Stabinger Viscometer. The viscometer has a dynamic viscosity range of 0.2 to 20 000 mPa.s, a kinematic viscosity range of 0.2 to 20 000 mm2/s and a density range of 0.65 to 3 g/cm3. The instrument is equipped with a maximum temperature range of +105oC and a minimum of 20oC below ambient. Instrument viscosity reproducibility is 0.35% of measured value and density reproducibility of 0.0005g/cm3.
Results and discussion
Table 1 presents a comparison of experimental densities and viscosities of all liquids at T = 293.15, 303.15, 313.15 and 323.15) K used in this work as well as accepted literature values.
Table 1: Comparison of experimental densities (r) and viscosities (h) with literature values
| Component | T = 293.15 | T = 303.15 | T = 313.15 | T = 323.15 | |||||
| r(g/cm3) | h(mPa.s) | r(g/cm3) | h(mPa.s) | r(g/cm3) | h(mPa.s) | r(g/cm3) | h(mPa.s) | ||
| m-xylene | ExperimentLiterature | 0.86400.8643[8]0.8638[17] | 0.55220.5580[13]0.719[17] | 0.85500.8556[8]0.8558[13]0.8559[17] | 0.50090.5580[13]0.650[17] | 0.84600.8469[8] | 0.4463 | 0.83800.8380[8] | 0.3949 |
| o-xylene | ExperimentLiterature | 0.87940.8798[8]0.8794[17] | 0.760360.920[17] | 0.87110.8714[8]0.8716[13]0.8714[17]0.8707[18] | 0.67250.6930[13]0.814[17] | 0.86260.8630[8] | 0.58924 | 0.85410.8545[8] | 0.51766 |
| p-Xylene | ExperimentLiterature | 0.86050.8609[8]0.8607[17] | 0.575620.566[13]0.752[17] | 0.85180.8523[8]0.8523[13]0.8524[17] | 0.520280.5660[13]0.676[17] | 0.84300.8435[8] | 0.45918 | 0.83420.8347[8] | 0.4046 |
| DMF | ExperimentLiterature | 0.94780.9436[7]0.9487[19] | 0.8370 | 0.93820.9336[7] | 0.7470 | 0.92860.9261[7] | 0.6652 | 0.91890.9149[7] | 0.5901 |
Experimental density (p), dynamic viscosity (η), at temperatures of (293.15, 303.15, 313.15 and 323.15 K) are presented in table 2. The table also lists deviation in viscosity, (Δη), excess molar volume, VE and excess Gibbs free energy of activation of viscous flow ΔG*E, for (m-xylene + N,N-dimethylformamide), (o-xylene + N,N-dimethylformamide), and (p-xylene + N,N-dimethylformamide) as a function of mole fraction.
Table 2:Experimental values of density r(g/cm3), viscosity h(mPa.s), deviation in viscosity Dh(mPa.s), excess molar volumes VE(cm3/mol), molar volume of mixture Vm(cm3/mol), excess Gibbs free energy of activation of viscous flow ΔG*E(J/mol), Grunberg-Nissan parameter (dʹ) and modified Kendall and Monroe viscosity correlation Ehm (mPa.s) with xylene (x1) and N,N-dimethylformamide (x2) at 293.15, 303.15, 313.15 and 323.15 K.
293.15K
| o-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9478 | 0.8370 | 0.0000 | 0.0000 | 77.1154 | 0.000 | 0.0000 | 0.0000 |
| 0.1003 | 0.9404 | 0.8459 | 0.0166 | -0.4046 | 81.3571 | 2.929 | 0.2239 | 0.0748 |
| 0.1970 | 0.9322 | 0.8510 | 0.0291 | -0.6332 | 85.6080 | 4.519 | 0.2244 | 0.1300 |
| 0.2951 | 0.9237 | 0.8497 | 0.0353 | -0.7704 | 90.0152 | 5.536 | 0.2086 | 0.1693 |
| 0.3935 | 0.9160 | 0.8465 | 0.0397 | -0.9110 | 94.4328 | 6.126 | 0.2055 | 0.1924 |
| 0.5010 | 0.9092 | 0.8390 | 0.0404 | -1.1551 | 99.1686 | 6.314 | 0.2019 | 0.1995 |
| 0.5879 | 0.9026 | 0.8279 | 0.0359 | -1.1744 | 103.1749 | 6.133 | 0.1878 | 0.1917 |
| 0.6875 | 0.8973 | 0.8171 | 0.0328 | -1.3959 | 107.5671 | 5.590 | 0.1951 | 0.1684 |
| 0.7872 | 0.8906 | 0.8000 | 0.0233 | -1.3900 | 112.1915 | 4.594 | 0.1811 | 0.1300 |
| 0.8994 | 0.8863 | 0.7841 | 0.0160 | -1.7290 | 117.0502 | 2.840 | 0.2325 | 0.0695 |
| 1.0000 | 0.8682 | 0.7604 | 0.0000 | 0.0000 | 123.4393 | 0.000 | 0.0000 | 0.0000 |
303.15K
| o-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9382 | 0.7470 | 0.0000 | 0.0000 | 77.9045 | 0.000 | 0.0000 | 0.0000 |
| 0.1003 | 0.9309 | 0.7527 | 0.0132 | -0.2430 | 82.1874 | 2.923 | 0.2013 | 0.0667 |
| 0.197 | 0.9228 | 0.7514 | 0.0191 | -0.3139 | 86.4800 | 4.467 | 0.1683 | 0.1158 |
| 0.2951 | 0.9145 | 0.7488 | 0.0238 | -0.2998 | 90.9207 | 5.485 | 0.1604 | 0.1507 |
| 0.3935 | 0.9068 | 0.7439 | 0.0263 | -0.2698 | 95.3909 | 6.071 | 0.1560 | 0.1711 |
| 0.501 | 0.9001 | 0.7361 | 0.0264 | -0.3404 | 100.1712 | 6.265 | 0.1519 | 0.1773 |
| 0.5879 | 0.8936 | 0.7281 | 0.0249 | -0.2188 | 104.2140 | 6.120 | 0.1492 | 0.1702 |
| 0.6875 | 0.8884 | 0.7170 | 0.0212 | -0.2824 | 108.6448 | 5.577 | 0.1454 | 0.1494 |
| 0.7872 | 0.8819 | 0.7033 | 0.0150 | -0.1277 | 113.2983 | 4.614 | 0.1340 | 0.1152 |
| 0.8994 | 0.8777 | 0.6929 | 0.0129 | -0.2918 | 118.1971 | 2.923 | 0.2133 | 0.0615 |
| 1.0000 | 0.8711 | 0.6725 | 0.0000 | 0.0000 | 123.0284 | 0.000 | 0.0000 | 0.0000 |
313.15 K
| o-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9286 | 0.6652 | 0.0000 | 0.0000 | 78.7099 | 0.000 | 0.0000 | 0.0000 |
| 0.1003 | 0.9214 | 0.6709 | 0.0133 | -0.2419 | 83.0348 | 2.945 | 0.2297 | 0.0593 |
| 0.197 | 0.9133 | 0.6672 | 0.0170 | -0.2999 | 87.3796 | 4.470 | 0.1702 | 0.1028 |
| 0.2951 | 0.9052 | 0.6627 | 0.0200 | -0.2912 | 91.8549 | 5.475 | 0.1543 | 0.1336 |
| 0.3935 | 0.8976 | 0.6566 | 0.0213 | -0.2576 | 96.3686 | 6.051 | 0.1453 | 0.1514 |
| 0.501 | 0.891 | 0.6485 | 0.0214 | -0.3266 | 101.1943 | 6.245 | 0.1416 | 0.1566 |
| 0.5879 | 0.8846 | 0.6390 | 0.0185 | -0.2031 | 105.2743 | 6.080 | 0.1287 | 0.1502 |
| 0.6875 | 0.8795 | 0.6306 | 0.0176 | -0.2681 | 109.7442 | 5.567 | 0.1395 | 0.1316 |
| 0.7872 | 0.8732 | 0.6168 | 0.0114 | -0.1246 | 114.4271 | 4.593 | 0.1194 | 0.1013 |
| 0.8994 | 0.869 | 0.6067 | 0.0100 | -0.2799 | 119.3804 | 2.908 | 0.1915 | 0.0540 |
| 1.0000 | 0.8626 | 0.5892 | 0.0000 | 0.0000 | 124.2407 | 0.000 | 0.0000 | 0.0000 |
323.15 K
| o-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9189 | 0.5901 | 0.0000 | 0.0000 | 79.5408 | 0.000 | 0.0000 | 0.0000 |
| 0.1003 | 0.9118 | 0.5960 | 0.0078 | -0.2392 | 83.9090 | 2.882 | 0.2563 | 0.0526 |
| 0.197 | 0.9039 | 0.5910 | 0.0045 | -0.3020 | 88.2883 | 4.311 | 0.1725 | 0.0910 |
| 0.2951 | 0.8959 | 0.5854 | 0.0007 | -0.2882 | 92.8084 | 5.219 | 0.1474 | 0.1182 |
| 0.3935 | 0.8884 | 0.5785 | -0.0044 | -0.2501 | 97.3666 | 5.701 | 0.1328 | 0.1339 |
| 0.501 | 0.8819 | 0.5708 | -0.0101 | -0.3164 | 102.2384 | 5.806 | 0.1291 | 0.1383 |
| 0.5879 | 0.8716 | 0.5874 | 0.0081 | 0.2977 | 106.8445 | 5.975 | 0.2988 | 0.1325 |
| 0.6875 | 0.8706 | 0.5534 | -0.0241 | -0.2559 | 110.8661 | 4.965 | 0.1200 | 0.1159 |
| 0.7872 | 0.8643 | 0.5412 | -0.0345 | -0.0964 | 115.6054 | 3.916 | 0.0986 | 0.0892 |
| 0.8994 | 0.8603 | 0.5332 | -0.0405 | -0.2683 | 120.5876 | 2.157 | 0.1795 | 0.0475 |
| 1.0000 | 0.8541 | 0.51771 | -0.0541 | 0.0000 | 125.4771 | -0.826 | 0.0000 | 0.0000 |
293.15 K
| m-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9461 | 0.8357 | 0.0000 | 0.0000 | 77.2329 | 0.0000 | 0.0000 | 0.0000 |
| 0.0894 | 0.938 | 0.7818 | -0.0286 | -0.2727 | 81.1498 | 2.3162 | -0.3639 | 0.0657 |
| 0.1962 | 0.9317 | 0.8246 | 0.0445 | -0.8202 | 85.6074 | 4.7632 | 0.4307 | 0.1220 |
| 0.2943 | 0.9182 | 0.7826 | 0.0303 | -0.5156 | 90.5093 | 5.6645 | 0.2711 | 0.1545 |
| 0.3922 | 0.9116 | 0.7974 | 0.0729 | -0.7862 | 94.8267 | 6.6890 | 0.4849 | 0.1705 |
| 0.4904 | 0.9006 | 0.7228 | 0.0261 | -0.5118 | 99.7031 | 6.4337 | 0.2323 | 0.1717 |
| 0.5885 | 0.886 | 0.6687 | -0.0002 | 0.3094 | 105.1217 | 6.0486 | 0.0864 | 0.1597 |
| 0.687 | 0.8805 | 0.6406 | -0.0003 | 0.1647 | 109.5931 | 5.5241 | 0.0874 | 0.1360 |
| 0.7785 | 0.8732 | 0.6122 | -0.0028 | 0.3661 | 114.0825 | 4.6647 | 0.0659 | 0.1049 |
| 0.8818 | 0.8711 | 0.5855 | -0.0002 | -0.1562 | 118.4013 | 3.1757 | 0.0919 | 0.0606 |
| 1.0000 | 0.8636 | 0.5522 | 0.0000 | 0.0000 | 124.0968 | 0.0000 | 0.0000 | 0.0000 |
303.15 K
| m-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9365 | 0.7676 | 0.0000 | 0.0000 | 78.0246 | -0.0005 | 0.0000 | 0.0000 |
| 0.0894 | 0.9284 | 0.6903 | -0.0534 | -0.2674 | 81.9889 | 1.9985 | -0.8345 | 0.0603 |
| 0.1962 | 0.9223 | 0.7180 | 0.0027 | -0.8318 | 86.4799 | 4.3388 | 0.1073 | 0.1119 |
| 0.2943 | 0.9089 | 0.6915 | 0.0024 | -0.5199 | 91.4354 | 5.3725 | 0.1020 | 0.1415 |
| 0.3922 | 0.9023 | 0.6984 | 0.0354 | -0.7853 | 95.8041 | 6.3346 | 0.3057 | 0.1559 |
| 0.4904 | 0.8914 | 0.6400 | 0.0032 | -0.5056 | 100.7322 | 6.1806 | 0.1101 | 0.1568 |
| 0.5885 | 0.8769 | 0.5961 | -0.0145 | 0.3313 | 106.2126 | 5.8628 | -0.0066 | 0.1457 |
| 0.687 | 0.8715 | 0.5718 | -0.0125 | 0.1810 | 110.7248 | 5.3591 | -0.0055 | 0.1239 |
| 0.7785 | 0.8643 | 0.5482 | -0.0118 | 0.3823 | 115.2573 | 4.5354 | -0.0249 | 0.0955 |
| 0.8818 | 0.8624 | 0.5284 | -0.0040 | -0.1689 | 119.5958 | 3.1202 | 0.0287 | 0.0551 |
| 1.0000 | 0.8549 | 0.5009 | 0.0000 | 0.0000 | 125.3597 | 0.0000 | 0.0000 | 0.0000 |
313.15 K
| m-Xylenex1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.8269 | 0.6823 | 0.0000 | 0.0000 | 88.3662 | 0.0000 | 0.0000 | 0.0000 |
| 0.0894 | 0.9189 | 0.6097 | -0.0515 | -8.9520 | 82.8366 | 1.0791 | -0.9158 | 0.0536 |
| 0.1962 | 0.9128 | 0.6250 | -0.0110 | -8.4972 | 87.3800 | 3.3985 | -0.0284 | 0.0995 |
| 0.2943 | 0.8995 | 0.6128 | 0.0000 | -7.2418 | 92.3909 | 4.6730 | 0.0842 | 0.1258 |
| 0.3922 | 0.893 | 0.6118 | 0.0221 | -6.5787 | 96.8018 | 5.6304 | 0.2411 | 0.1387 |
| 0.4904 | 0.8822 | 0.5667 | 0.0002 | -5.3572 | 101.7826 | 5.6568 | 0.0904 | 0.1395 |
| 0.5885 | 0.8678 | 0.5274 | -0.0160 | -3.5690 | 107.3264 | 5.4234 | -0.0316 | 0.1297 |
| 0.687 | 0.8625 | 0.5062 | -0.0139 | -2.7859 | 111.8802 | 5.0157 | -0.0319 | 0.1103 |
| 0.7785 | 0.8554 | 0.4849 | -0.0137 | -1.7126 | 116.4565 | 4.2700 | -0.0640 | 0.0850 |
| 0.8818 | 0.8535 | 0.4689 | -0.0053 | -1.2807 | 120.8429 | 2.9783 | -0.0068 | 0.0491 |
| 1.0000 | 0.8462 | 0.4463 | 0.0000 | 0.0000 | 126.6485 | 0.0000 | 0.0000 | 0.0000 |
323.15 K
| m-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9172 | 0.6057 | 0.0000 | 0.0000 | 79.6664 | -0.0003 | 0.0000 | 0.0000 |
| 0.0894 | 0.9093 | 0.5380 | -0.0488 | -0.2621 | 83.7111 | 1.8975 | -0.9847 | 0.0476 |
| 0.1962 | 0.9032 | 0.5598 | -0.0044 | -0.8097 | 88.3087 | 4.2462 | 0.0345 | 0.0882 |
| 0.2943 | 0.8901 | 0.5419 | -0.0015 | -0.4779 | 93.3666 | 5.3261 | 0.0730 | 0.1116 |
| 0.3922 | 0.8836 | 0.5355 | 0.0128 | -0.7293 | 97.8316 | 6.1104 | 0.1901 | 0.1229 |
| 0.4904 | 0.873 | 0.4998 | -0.0021 | -0.4365 | 102.8553 | 6.1117 | 0.0745 | 0.1236 |
| 0.5885 | 0.8587 | 0.4650 | -0.0162 | 0.4460 | 108.4638 | 5.7880 | -0.0473 | 0.1148 |
| 0.687 | 0.8534 | 0.4467 | -0.0137 | 0.3102 | 113.0732 | 5.2994 | -0.0435 | 0.0976 |
| 0.7785 | 0.8464 | 0.4275 | -0.0134 | 0.5236 | 117.6948 | 4.4651 | -0.0800 | 0.0752 |
| 0.8818 | 0.8447 | 0.4142 | -0.0049 | -0.0459 | 122.1018 | 3.0951 | -0.0097 | 0.0434 |
| 1.0000 | 0.8383 | 0.3941 | 0.0000 | 0.0000 | 127.8421 | -0.0002 | 0.0000 | 0.0000 |
293.15 K
| p-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9478 | 0.8370 | 0.0000 | 0.0000 | 77.0943 | 0.0000 | 0.0000 | 0.0000 |
| 0.0896 | 0.9455 | 0.9666 | 0.1530 | -0.8325 | 80.5133 | 3.9870 | 0.1908 | 0.0661 |
| 0.1961 | 0.9338 | 0.9375 | 0.1517 | -0.9879 | 85.4112 | 5.7378 | 0.2777 | 0.1231 |
| 0.2942 | 0.9181 | 0.7689 | 0.0088 | -0.5385 | 90.5154 | 5.4089 | -0.4828 | 0.1564 |
| 0.3923 | 0.9116 | 0.8533 | 0.1188 | -0.8783 | 94.8304 | 7.1070 | 0.2435 | 0.1732 |
| 0.4904 | 0.9017 | 0.8315 | 0.1227 | -0.7821 | 99.5815 | 7.4054 | 0.3456 | 0.1751 |
| 0.5885 | 0.8856 | 0.6851 | 0.0019 | 0.1508 | 105.1692 | 6.0339 | -0.2194 | 0.1635 |
| 0.6866 | 0.8788 | 0.7203 | 0.0628 | 0.1164 | 109.7896 | 6.2598 | 0.2377 | 0.1400 |
| 0.7847 | 0.8715 | 0.6254 | -0.0065 | 0.2197 | 114.5476 | 4.5132 | -0.2128 | 0.1058 |
| 0.8828 | 0.8642 | 0.6073 | 0.0010 | 0.4033 | 119.3861 | 3.1999 | -0.1082 | 0.0624 |
| 1.0000 | 0.8605 | 0.5756 | 0.0000 | 0.0000 | 124.5439 | 0.0000 | 0.0000 | 0.0000 |
303.15 K
| p-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9382 | 0.7470 | 0.0000 | 0.0000 | 77.8832 | 0.0001 | 0.0000 | 0.0000 |
| 0.0896 | 0.9361 | 0.8311 | 0.1044 | -0.7538 | 81.3218 | 3.6728 | -1.5502 | 0.0591 |
| 0.1961 | 0.9243 | 0.8047 | 0.1021 | -0.7698 | 86.2891 | 5.4079 | -0.5659 | 0.1101 |
| 0.2942 | 0.9087 | 0.6743 | -0.0060 | -0.1974 | 91.4518 | 5.2546 | -0.9724 | 0.1401 |
| 0.3923 | 0.9023 | 0.7412 | 0.0831 | -0.4315 | 95.8079 | 6.8702 | -0.1814 | 0.1553 |
| 0.4904 | 0.8924 | 0.7189 | 0.0831 | -0.2103 | 100.6193 | 7.1287 | -0.0383 | 0.1572 |
| 0.5885 | 0.8765 | 0.6082 | -0.0054 | 0.8414 | 106.2611 | 5.9729 | -0.4659 | 0.1470 |
| 0.6866 | 0.8698 | 0.6307 | 0.0394 | 0.9156 | 110.9256 | 6.0819 | -0.0571 | 0.1260 |
| 0.7847 | 0.8626 | 0.5569 | -0.0122 | 1.1294 | 115.7295 | 4.4712 | -0.4307 | 0.0954 |
| 0.8828 | 0.8553 | 0.5429 | -0.0040 | 1.4381 | 120.6284 | 3.1889 | -0.3295 | 0.0563 |
| 1.0000 | 0.8596 | 0.5203 | 0.0000 | 0.0000 | 124.6743 | 0.0005 | 0.0000 | 0.0000 |
313.15 K
| p-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9286 | 0.6652 | 0.0000 | 0.0000 | 78.6884 | 0.0005 | 0.0000 | 0.0000 |
| 0.0896 | 0.9265 | 0.7202 | 0.0735 | -0.8642 | 82.1645 | 3.4465 | -3.1684 | 0.0526 |
| 0.1961 | 0.9148 | 0.6949 | 0.0701 | -1.0024 | 87.1852 | 5.1533 | -1.3404 | 0.0979 |
| 0.2942 | 0.8993 | 0.5910 | -0.0136 | -0.5320 | 92.4077 | 5.1232 | -1.4302 | 0.1244 |
| 0.3923 | 0.8929 | 0.6478 | 0.0634 | -0.8753 | 96.8165 | 6.7160 | -0.5404 | 0.1379 |
| 0.4904 | 0.8831 | 0.6246 | 0.0604 | -0.7649 | 101.6789 | 6.9234 | -0.3563 | 0.1394 |
| 0.5885 | 0.8674 | 0.5365 | -0.0075 | 0.1800 | 107.3759 | 5.8942 | -0.6802 | 0.1303 |
| 0.6866 | 0.8608 | 0.5486 | 0.0248 | 0.1374 | 112.0853 | 5.8859 | -0.3068 | 0.1115 |
| 0.7847 | 0.8536 | 0.4910 | -0.0126 | 0.2497 | 116.9497 | 4.3880 | -0.5961 | 0.0844 |
| 0.8828 | 0.8464 | 0.4777 | -0.0056 | 0.4448 | 121.8968 | 3.0899 | -0.4990 | 0.0497 |
| 1.0000 | 0.843 | 0.4592 | 0.0000 | 0.0000 | 127.1293 | 0.0000 | 0.0000 | 0.0000 |
323.15 K
| p-Xylene x1 | r(g/cm3) | h(mPa.s) | ∆h(mPa.s) | VE(cm3/mol) | Vm(cm3/mol) | ∆G*E(J/mol) | d’ | Ehm (mPa.s) |
| 0.0000 | 0.9189 | 0.5901 | 0.0000 | 0.0000 | 79.5190 | -0.0001 | 0.0000 | 0.0000 |
| 0.0896 | 0.9617 | 0.6240 | 0.0505 | -4.7471 | 79.1579 | 2.8573 | -4.7885 | 0.0466 |
| 0.1961 | 0.9052 | 0.5986 | 0.0448 | -1.0085 | 88.1098 | 4.9196 | -2.1299 | 0.0867 |
| 0.2942 | 0.8899 | 0.5502 | 0.0147 | -0.5367 | 93.3838 | 5.5413 | -1.5952 | 0.1102 |
| 0.3923 | 0.8834 | 0.5648 | 0.0474 | -0.8650 | 97.8576 | 6.5951 | -0.9076 | 0.1220 |
| 0.4904 | 0.8737 | 0.5425 | 0.0434 | -0.7519 | 102.7729 | 6.7778 | -0.6715 | 0.1233 |
| 0.5885 | 0.8582 | 0.4719 | -0.0091 | 0.2001 | 108.5270 | 5.8583 | -0.9024 | 0.1151 |
| 0.6866 | 0.8517 | 0.4737 | 0.0109 | 0.1539 | 113.2829 | 5.7004 | -0.5855 | 0.0985 |
| 0.7847 | 0.8446 | 0.4317 | -0.0129 | 0.2648 | 118.1959 | 4.3591 | -0.7699 | 0.0745 |
| 0.8828 | 0.8374 | 0.4100 | -0.0164 | 0.4736 | 123.2069 | 2.8658 | -0.8971 | 0.0439 |
| 1.0000 | 0.8342 | 0.4046 | 0.0000 | 0.0000 | 128.4704 | 0.0000 | 0.0000 | 0.0000 |
To investigate the molecular interaction between N,N-dimethylformamide and m-xylene, o-xylene and p-xylene, viscosity deviation, Δη, excess molar volumes VE and excess Gibbs free energy of activation of viscous flow, ΔG*E, have been evaluated from experimental density and viscosity using the equations 1 and 2 respectively.
wherex1 and x2 are the mole fractions calculated from mass fractions. M1 and M2 are molar masses, p1 and p2 are densities, η1 and η2 are viscosities of pure components 1 and 2 respectively. pm and ηm are the density and viscosity of the mixture.
The excess Gibbs free energy of activation of viscous flow were obtained from equation 3.
whereR is the universal constant of gases, T is the absolute temperature, V1 and V2 are the molar volumes of component 1 and 2, x1 and x2 represents the mole fraction of component 1 and 2. Vm is obtained from equation 4. η1, η2 and ηm are the viscosity of component 1 and 2 and mixture respectively.
The values of VE, Δηand DG*E were correlated by a Redlich-Kister16 type polynomial, equation
The values of the parameters Ak, are obtained by fitting the equation to the experimental values with the least-squares method. The correlated results for excess molar volume, viscosity deviation and excess Gibbs free energy of activation of viscous flow are presented in Table 3. The standard deviation s(ΔY) is calculated from equation 6.
 |
Table3: Adjustable parameters Ai, with standard deviations s(DY), for deviation in viscosity (Dh), Excess volume (VE), and Excess Gibbs free energy (DG*E), for binary mixtures at various temperatures. Click here to View table |
whereDY is the excess volume, VE, deviation in viscosity Dh, and excess Gibbs free energy of activation of viscous flow, ΔG*E. The subscript expt and calc represents the experimental and calculated values respectively. N and n are the number of experimental data points and the number of coefficients in the Redlich-Kister polynomial equation.
Kendall and Monroe14 derived equation 7 for analyzing the viscosity of binary mixtures based on zero adjustable parameter.
whereEnm is a modified Kendall-Monroe10,15 equation, 8.
The predictive ability of some selected viscosity models such as the one parameter model of Frenkel20 equation 9 and Hind21 equation 10, apply to the studied binary mixtures.
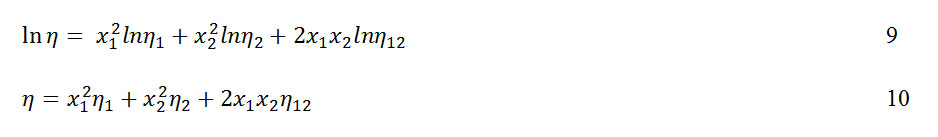
wheren12 is a constant attributed to unlike pair interactions. Its value is obtained from equation
Grunberg and Nissan22 formulated equation 12 to determine the molecular interactions leading to viscosity changes with one parameter to estimate the dynamic viscosity of binary liquid mixtures.
wheredʹ is an interaction parameter which is a function of the composition and temperature of binary liquid mixture.
The correlating ability of equations 8, 9, 10 and 12 were tested by calculating the average percentage deviations (APD) between the experimental and the calculated viscosity as shown in equation 14.
wherenexpt and ncalc represent the viscosity of experimental and calculated data, N is the number of experimental data points.
Deviations in viscosity (Δn) for the binary system N,N-dimethylformamide (DMF) and o-xylene, m-xylene and p-xylene at 293.15, 303.15, 313.15 and 323.15 K, plotted against mole fraction with the curve calculated from Redlich-Kister equation are presented in figure 1(a-c). Viscosity deviations for N,N-dimethylformamide (DMF) with o-xylene and p-xylene are positive while that of N,N-dimethylformamide (DMF) and m-xylene were found to be both positive and negative. Deviation in viscosity are related to molecular interactions such as hydrogen bonding, charge transfer interactions and physical interactions of dispersion forces or weak dipole-dipole interactions between compounds in a mixture.23 The reason why a positive VE and a negative Dh may result in a binary system is because of a disruption in associated molecules while a negative VE and positive Δn is caused by an association or compound formation between components.24 In a system where positive values of Δn are observed, specific interactions cause complex formation. When negative values of Δn are observed, dispersion forces are therefore dominant.23 In our system, positive deviations in Δn show a complex formation between N,N-dimethylformamideand o-xylene, m-xylene and p-xylene while negative deviations in Dh, have resulted as a consequence of disruption in hydrogen bonding and increase in temperature as observed by other researchers.24N,N-dimethylformamide has a lone pair of electron on the oxygen atom making it an electron donor while the xylenes on the other hand are electron acceptors. It is therefore possible for hydrogen bonding interactions between N,N-dimethylformamide and the xylenes to occur resulting in complex formation.8
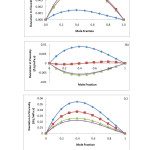 |
Fig1: Plots of deviation in viscosity (Dh) against mole fraction for the system (a) o-xylene (1) + N,N-dimethylformamide(2); (b) m-xylene (1) + N,N-dimethylformamide(2); (c) p-xylene (1) + N,N-dimethylformamide(2) at different temperatures: ¨, 293.15 K; ■, 303.15 K;▲, 313.15 K; x, 323.15 K. The solid line represents the corresponding correlation by the Redlich-Kister equation. Click here to View Figure |
The plots of excess molar volume (VE) against mole fraction at 293.15, 303.15, 313.15 and 323.15 K for N,N-dimethylformamide (DMF) and o-xylene, m-xylene and p-xylene calculated from Redlich-Kister equation are presented in figure 2 (a-c). Excess parameters associated with a liquid mixture are a quantitative measure of deviation in the behavior of the liquid mixture from ideality.10These functions are found to be sensitive towards the intermolecular forces and also on the difference in size and shape of the molecules. Excess volumes of liquid mixtures reflect the result of different contributions arising from structural changes undergone by the pure cosolvent. Positive contributions arise from breakup interactions between molecules namely, the rupture of hydrogen bonded chains and the loosening of dipole interactions.25 Excess molar volume were found to be both positive and negative for the binary system between N,N-dimethylformamide and o-xylene, figure 2(a). The binary system of N,N-dimethylformamide with m-xylene and p-xylene, figures 2(b-c), excess molar volumes are negative. For the binary systems, N,N-dimethylformamide with m-xylene and p-xylene, that form a negative deviation, the curves are not shifted in a regular manner with increase in temperature. For the binary system of N,N-dimethylformamide and o-xylene, positive deviation is observed at 293.15 K only. At the other temperatures, deviations are negative. Mixing N,N-dimethylformamide (DMF) with o-xylene, m-xylene and p-xylene may have resulted in complex formation leading to a volume contraction.
 |
Fig2: Plots of Excess molar volume (VE) against mole fraction for the system(a) o-xylene (1) + N,N-dimethylformamide(2); (b) m-xylene (1) + N,N-dimethylformamide (2); (c) p-xylene (1) + N,N-dimethylformamide (2) at different temperatures: ¨, 293.15 K; ■, 303.15 K;▲, 313.15 K; x, 323.15 K. The solid line represents the corresponding correlation by the Redlich-Kister equation. Click here to View Figure |
Excess Gibbs free energy of activation of viscous flow (ΔG*E) against mole fraction at 293.15, 303.15, 313.15 and 323.15 K for N,N-dimethylformamide (DMF) and o-xylene, m-xylene and p-xylene calculated from Redlich-Kister equation are presented in figure 3(a-c). Excess properties provide information about the molecular interactions and macroscopic behavior of fluid mixtures which can be used to test and improve thermodynamic models for calculating and predicting fluid phase equilibria.10 The magnitude of ΔG*E represents the strength of interaction between unlike molecules.26 Excess Gibbs free energy of activation of viscous flow are positive at all experimental temperatures.
 |
Fig3: Plots of Excess Gibbs free energy of activation of viscous flow (DG*E) against mole fraction for the system(a) o-xylene (1) + N,N-dimethylformamide(2); (b) m-xylene (1) + N,N-dimethylformamide (2); (c) p-xylene (1) + N,N-dimethylformamide (2) at different temperatures: ¨, 293.15 K; ■, 303.15 K;▲, 313.15 K; x, 323.15 K. The solid line represents the corresponding correlation by the Redlich-Kister equation. Click here to View Figure |
A comparison of experimental thermodynamic data of multicomponent mixtures with that calculated by means of various predictive methods is very useful from different points of view: (i) it suggests which model is more appropriate to the characteristics of the system, (ii) it may indicate which parameters should be improved when the model involves group contributions and (iii) it may allow the identification of some model as a convenient reference for the interpretation of the deviations observed.10,27 The viscosity data have been correlated with semi-empirical equations of modified Kendall and Monroe, Frenkel, Hind, and Grunberg-Nissan. The values of the Grunberg and Nissan constant (dʹ) and modified Kendall-Monroe (Ehm) for all systems under study are presented in table 2. Grunberg-Nissan interaction parameters are both positive and negative while the modified Kendall-Monroe viscosity correlation data are all positive. Plots for the modified Kendall-Monroe viscosity correlation are presented in Figure 4(a-c). Plots of modified Kendall-Monroe viscosity correlation at different temperatures show a decrease in viscosity with increase in temperature. The value of Frenkel and Hind are presented in Table 4. Positive and negative Grunberg-Nissan parameters indicate the presence of both strong and weak interactions between unlike molecules.28
 |
Fig4: Plots of modified Kendall and Monroe viscosity correlation Ehm (mPa.s) against mole fraction for the system(a) o-xylene (1) + N,N-dimethylformamide(2); (b) m-xylene (1) + N,N-dimethylformamide (2); (c) p-xylene (1) + N,N-dimethylformamide (2) at different temperatures: ¨, 293.15 K; ■, 303.15 K;▲, 313.15 K; x, 323.15 K. Click here to View Figure |
Table 4: Fitting parameters with Average Percentage Deviations (APD) for binary mixtures at various temperatures.
| Temperature |
Frenkel |
Hind |
||
|
K |
η12 |
APD |
η12 |
APD |
|
o-Xylene (1) + N,N-dimethylformamide (2) |
||||
| 293.15 | 0.7987 | 11.58 | 0.7987 | 0.27 |
| 303.15 | 0.7098 | 13.39 | 0.7098 | 0.21 |
| 313.15 | 0.6272 | 15.71 | 0.6272 | 0.19 |
| 323.15 | 0.5539 | 18.57 | 0.5539 | 0.22 |
|
m-Xylene (1) + N,N-dimethylformamide (2) |
||||
| 293.15 | 0.6940 | 13.91 | 0.6940 | 0.17 |
| 303.15 | 0.6343 | 15.81 | 0.6343 | -0.07 |
| 313.15 | 0.5643 | 18.60 | 0.5643 | -0.13 |
| 323.15 | 0.4999 | 22.09 | 0.4999 | -0.16 |
|
p-Xylene (1) + N,N-dimethylformamide (2) |
||||
| 293.15 | 0.7063 | 13.33 | 0.7063 | 0.66 |
| 303.15 | 0.6337 | 15.39 | 0.6337 | 0.47 |
| 313.15 | 0.5622 | 18.15 | 0.5622 | 0.36 |
| 323.15 | 0.4974 | 21.59 | 0.4974 | 0.29 |
Conclusion
Density and viscosity for the binary mixtures of N,N-dimethylformamide with o-xylene, m-xylene and p-xylene are presented as a function of mixture composition at 293.15, 303.15, 313.15, and 323.15 K. These results have been employed to calculate deviations in viscosity, excess molar volume and excess Gibbs free energy of activation of viscous flow. The study has shown that there are intermolecular interactions between the components of the systemN,N-dimethylformamide (DMF) with o-xylene, m-xylene and p-xylene, leading to hydrogen bonds and complex formation. A comparison of experimental thermodynamic data with calculated data by means of various predictive methods are presented resulting in positive and negative correlations.
Acknowledgement
This work was supported by a research grant from the Faculty of Applied and Computer Science Research and Publications Committee of Vaal University of Technology, Vanderbijlpark, Republic of South Africa.
References
- Xiao-Hui, R.;Hai-Jun, W.; Yan-Yan, S.; Wei-Bo, D.Struct. Chem. 2008,19, 233 – 238.
- Nain, A. K.;Sharma,R.J. Chem. Thermodyn.2013,58,36
- Clara, R. A.;Marigliano, A. C. G.;Campos, V. V.;Solimo,H. N. Fluid Phase Equilib. 2010, 293,151
- Mohammad, A. A.;Alkhald, K. H. A. E.;AlTuwaim, M. S.;Al-Jimaz,A. S.J. Chem. Thermodyn.2013,56,106
- Weissermel, K.;Arpe,H.-J. Industrial Organic Chemistry: Important raw materials and intermediates. Wiley-VCH Verlag GmbH. Weinheim, Germany, 1997, p. 45
- Hao, Y.;Zhang, S.;Yao,K. J. Chem. Eng. Data.2012, 57,1244
- Chen, B.;Liu,W.J. Chem. Thermodyn. 2007, 39,192
- Gu, Y.;Wu,J.J. Mol. Liq.2008,137,165
- Singh, S.;Sethi,B. P. S.;Katyal, R. C.;Rattan,V. K. J. Chem. Eng. Data.2005,50,125
- Dikio, E. D.;Vilakazi, G.;Ngoy,P.J. Mol. Liq.2013, 177,190
- Bhatia, S. C.;Rani, R.;Bhatia,R.J. Mol. Liq. 2011, 159,132
- Yan, Q.;Zhiguo, D.I.;Youguang, M.A.;PeishengM.A.;Shuquian,X. Chin. J. Chem. Eng.2010,183,446
- Rathnam, M.V.;Mohite, S.;Kumar,M.S.S.Ind. J. Chem. Tech.2008,157, 409 – 412.
- Kendall, J.;Monroe,K. P.J. Am. Chem. Soc.1917, 39(9), 1787 – 1802.
- Dikio, E. D.;Nelana, S. M.;Isabirye, D. A.;Ebenso,E. E.Int. J. Electrochem. Sci.2012, 7(6), 11101
- Redlich, O.;Kister,A. T.Ind. Eng. Chem.1948, 40(2), 345
- Serrano, L.;Silva, J. A.;Farelo,F.J. Chem. Eng. Data. 1990, 35, 288
- Narendra, K.;Narayanamurthy, P.;Srinivasu,C.E. J. Chem. 2010, 7(3), 927
- Lange’s Handbook of Chemistry 10th edition, p. 1525
- FrenkelYa. I. Theory of the Viscosity of liquid mixtures. Petroleum (London) 9, 1946, p. 27
- Hind, R. K.;McLaughlin, E.;Ubbelohde,A. R.Trans Faraday Soc.1960,56, 328
- Grunberg, L.;Nissan,A. H.Nature1949, 164, 799
- Živković, E. M.;Kijevčanin, M. Lj.;Radović, I. R.;Šerbanović, S. P.;Djordjević,B. D. Fluid Phase Equilib.2010, 299, 191
- Chowdhury, M. A.;Majid, M. A.;Saleh,M. A.J. Chem. Thermodyn. 2001, 33, 347
- El-Banna,M. M.Can. J. Chem.1997,75, 1890
- Shafiq, M.;Asif, S. M.; Farooqui,A. J. Biochem. Pharm. Res.2011, 1(2), 419
- Parveen, S.;Yasmin, M.;Gupta, M.;Shukla,J. P.Int. J. Thermodyn. 2010,13(2), 59
- Sathyanarayana, B.;Ranjithkumar, B.;Jyostna, T. S.;Satyanarayana,N.J. Chem. Thermodyn.2007, 39, 16

This work is licensed under a Creative Commons Attribution 4.0 International License.