Antimicrobial relevance of Cu (II) complexes of thiophene-2-glyoxal-p-halogen substituted Anils
Fentahun Adamu1, Sanidhya Upadhyay2, R.K.Upadhyay1*
1Chemistry of Department College of Natural and Computational Science Jigjiga University, Ethiopia 2Ranbaxy Research Laboretories, R & DV Department Plot No. 20, Section 18,Udyog, Gurgaon, India
DOI : http://dx.doi.org/10.13005/ojc/300314
Article Received on :
Article Accepted on :
Article Published : 17 Sep 2014
Isomeric complexes of, p-chloro, p- bromo, and p-iodo – anils of 2-thiophene glyoxal ligands have been synthesized and characterized by metal analysis, molecular weight, infrared and UV-Visible spectroscopy, molar conductance and magnetic measurements. All non electrolytic complexes are square planar paramagnetics. Among all of the compounds only p-bromo and p-iodo-anils of 2-thiophene glyoxal exhibited good anti fungal activity against both test fungi but all products showed moderate to good bactericidal effect against S.aures and X.holicicola bacteria.
KEYWORDS:Complexes; anils; antimicrobial; paramagnetics; 2- thiophene glyoxal
Download this article as:| Copy the following to cite this article: Adamu F, Upadhyay S, Upadhyay R. K. Antimicrobial relevance of Cu (II) complexes of thiophene-2-glyoxal-p-halogen substituted anils. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Adamu F, Upadhyay S, Upadhyay R. K. Antimicrobial relevance of Cu (II) complexes of thiophene-2-glyoxal-p-halogen substituted anils. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4735 |
INTRODUCTION
Among the variety of organic compounds Schiff’s bases have earned highest popularity owing to their preparative accessibility and multifarious applications, viz. in chemistry as analytical reagents [1,2], as starting materials in the synthesis of hetercycles [3-5] and as novel ligands in forming complexes of unusual stereochemistries [6,7] and isomeric structures [6,8,9], in agriculture as herbicides [10], in industries as dyes [11] and catalytic agents [11] and in medical science as antimicrobial, anticancer, antitumor, antimalarial, antituberculosis, analgesic etc. agents [13-15].
High versality of Schiff’s bases in exhibiting wide spectrum of biological properties, enhanced biological properties of metal-based organic compounds observed generally and alarming problem of multi-drug resistance of several microbes, bacteria and fungi, impelled us to design some copper (II) – based Schiff,s bases antimicrobials with the view to addess the problem of multi-drug resistance of a few selected bacteria and fungi. Therefore in the present communication we report synthesis, structural characterization and antimicrobial potential against Staphylococcus aureus and Xanthomonas holicicola bacteria, and Fusarium oxysporum and Aspergillus niger fungi in vitro of copper (II) complexes of p-chloro-, p-bromo and p-iodo-anils of 2-thiophene glyoxal [16], abbreviated as TGCA, TGBA and TGIA respectively.
MATERIALS AND METHOD
Melting points determined in open glass capillaries are uncorrected. Metal contents of complexes were estimated by atomic absorption spectroscopy on Buck Scientific Model 210VGB. Molecular weights were determined by Rast’s micro method using camphor as solvent. IR spectra were recorded in 4000 – 200 cm-1 range on Perkin Elmer R (FTIR) spectro photo meter in nujol and 1HNMR spectra were recorded in CDCl3 on Bruker Ultra shield TM- 400 spectrometer using TMS as internal standard. Electronic spectral measurements were done on UV/Vis- SP65 SYANO spectro photometer in acetone, whereas conducto metric measurements were done on Jenway digital conductivity meter in acetone. Magnetic susceptibility of powdered samples was measured with MSB-AUTO, (Sherwood Scientific) magnetic balance.
Synthesis of thiophene -2-glyoxal –p-halogen substituted anils
All the three ketoanils, TGCA, TGBA and TGIA, were synthesized by reported procedure [16].On mixing saturated solution of thiophene-2-glyoxal (0.04 mol) with saturated solution of each of p-chloro-, p- bromo- and p-iodo- anilines (0.04 mol) in diethylether with continuous stirring and allowing the reaction mixtures to stand for half an hour at room temperature, precipitates of products obtained were filtered out, washed with ether and dried in air.
Thiophene -2- glyoxal was prepared by oxidation of 2-acetyl thiophene (0.5mol) with an equimolar quantity of selenium dioxide (0.5mol) in ethyl alcohol by refluxing as reported [16].
Synthesis of complexes
For the synthesis of complexes saturated solution containing 0.01 mol of each of copper salts, CuCl2.2H2O and Cu (NO3)2.3H2O, was mixed with saturated solution of each ketoanil ligand (0.015mol) in acetone and reaction mixtures were refluxed for about 1h. On cooling the reaction mixtures precipitated products were filtered out, washed with benzene/toluene, dried and finally washed with water and dried in air.
Homogeneity of the products was tested by thin-layer chromatography. All the three ketoanils exhibited single spot migration whereas complexes displayed two spots with different Rf values in several solvents. Owing to maximum Rf difference of components of binary mixtures in benzene and chloroform (low Rf 0.00 and high Rf 0.83-0.97) they were separated by column chromatography with these solvents using silica gel (BHD 60 mesh). Low Rf products were eluted with acetone and on evaporation of solvents from eluates solids were recovered and weighed. Quantity of high Rf products of CuCl2– TGCA and Cu (NO3)2-TGCA being very little, they could not be processed for structural and antimicrobial investigations. In the synthesis of compounds BDH/ Merck laboratory reagents were used as supplied whereas in chromatographic work HPLC grade solvents were used.
Evaluation of antimicrobial activities
Antimicrobial, fungicidal and bactericidal, activities of all the compounds were tested in vitro on S aureus and X. holicicola bacteria and F. oxysporum and A. niger fungi, using disc diffusion method. Isolates of both bacteria and fungi were cultured on nutrient agar (NA) and potato dextrose agar (PDA) media respectively. Aliquots of 5, 10 and 20 µl of 5mg/ml concentrated solutions of samples in acetone were pipetted to the discs in three replications of each and transferred to NA plates seeded with bacteria and PDA seeded with spore suspension of test fungi. For antifungal studies petri dishes were incubated at 26 0C for 5-7days whereas petri dishes containing bacteria for loaded discs incubated a 370C for A.ureus and at 280C X.holicicola for 24 h.
RESULTS AND DISCUSSION
Structural studies
Although synthesis and structures of TGCA, TGBA and TGIA have been reported [10,14] earlier but for further verification of reported structural results their UV, IR and 1HNMR spectra have been recorded and analyzed. UV: C=O, ca. 220 nm (π→ π*) ca. 327 nm (n→ π*) ; C=N, ca. 243 nm (π→ π*), 1HNMR : δ 7.25- 8.20 (1H, CH=N) ; δ 7.1-7.9 (3H, thienyl ring); δ 6.43- 7.74 (4H, benzene ring).
In all the three ketoanils, halogen substituent in para position being electron donor could transfer electron density to carbonyl oxygen which may lead to the molecular rearrangement of the benzenoid structures I to quinonoid form II. After acquiring some negative charge quinonoid oxygen is more activated for coordination than benzenoid oxygen (structure I). Therefore structure II could offer three coordinating sites, carbonyl oxygen, thienyl sulphur and azomethine nitrogen (Fig.,1).
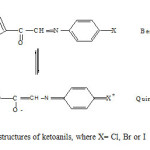 |
Fig1: Tautomeric structures of ketoanils, where X= Cl, Br or I Click here to View Figure |
Formulation of complexes has been done in accordance with their metal analysis, molecular weight and conductance data (Tabe-1).
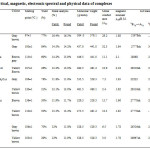 |
Table1: Analytical, magnetic, electronic spectral and physical data of complexes Click here to View table |
In order to study the binding modes of ketoanil ligands in the complexes, IR spectra of the free ligands are compared with their respective metal complexes. Perusal of infrared spectra of ligands and their respective complexes reveals that stretching bands corresponding to C=C(aromatic), 1:4 disubstitution , and C-Cl, C-Br and C-I groups of TGCA,TGBA and TGIA occurring at 1615, 1447 & 1436 cm-1, 820 cm-1 and 632 cm-1, 1587, 1470 & 1454 cm-1, 804 cm-1 and 550 cm-1 and 1584 & 1449 cm-1, 807 cm-1 and 500 cm-1 respectively are shifted to higher frequency side at ca. 1594, 1521 & 1462 cm-1, ca. 1585, 1498 & 1457 cm-1, ca. 828 cm-1 and ca. 552 cm-1, and ca. 1582, 1460 & 1452 cm-1, ca. 826 cm-1 and ca. 515 cm-1 of the spectra of their respective complexes.. This increase in frequencies of these groups of ligands on complexation clearly indicates presence of quinonoid structure (II) of the ligands in their complexes. TheυC=O band of TGCA, TGBA and TGIA occurring at 1775 cm-1,1675 cm-1 and 1650 cm-1 is observed at ca. 1688 cm-1, ca.1619 cm-1 and ca.1619 cm-1 in their complexes respectively. The lowering in carbonyl group stretching frequency of ligands on complexation with copper is the strong evidence of coordination of carbonyl group with the metal. A new band in 475-530 cm-1 region of complex spectra, attributed to Cu-O stretching, supports the coordination of carbonyl group of ligands. The lowering in υC=N frequency of TGCA from 1675 cm-1 to ca. 1594 cm-1 in its chloro-and nitro complexes, of TGBA from 1616 cm-1 to ca.1587 cm-1 in their low Rf (a) complexes and of TGIA from 1613 cm-1 to ca. 1587 cm-1 in its low Rf (a) complexes and appearance of a new band of Cu-N stretching in 405 – 431 cm-1 range of spectra of these complexes indicate presence of azomethine group [17 ] nitrogen in the coordination zone of Cu(II) in them. The undisplaced position of υC-S-C band of ligands in these complexes reveals absence of thienyl sulphur in coordination sphere of metal.
In high-Rf (b) complexes of TGBA and TGIA decrease in υC-S-C frequency of ligands from 725 cm-1 and 721 cm-1 to ca.686 cm-1 and ca.664 cm-1 respectively and appearance of a new low frequency band in 238-250 cm-1 range of, υCu-S in complex spectra are suggestive of coordination of thienyl sulphur [17,18 ] whereas undisturbed C=N stretching frequency of ligands in these complexes indicates non- participation of azomethine group in coordination.
A medium intensity band, characteristics of υCu-Cl, occurring in 348-385 cm-1 region of all the chloro complexes clearly exhibit coordination of monodentate chlorine. All the nitrato complexes display four bands at ca.1301 cm-1, ca.1037 cm-1, ca.1461 cm-1 and ca.885 cm-1 corresponding to [19] υNO2 symmetric, υNO, υNO2 asymmetric and ρr NO2 asymmetric out- of -plane respectively only out of the expected six bands and one pairs of combination bands at ca.1771 cm-1 and ca.1748 cm-1 except Cu(TGCA)(NO3)2 which displays two pairs of bands as expected at 1775 cm-1 and 1750 cm-1, and 2434 cm-1 and 2293 cm-1; the energy separation of first two bands and last two of these bands is ca.24 cm-1 and 141 cm–.1 These results and a new additional band of υCu-N in 468-498
cm-1 region of spectra lead us to propose monoligancy of nitrato ion through its nitrogen.
Both isomeric complexes of CuCl2-TGBA display symmetric and antisymmetric stretching and bending vibrations of lattice water [19]at 3444 cm-1 and ca.1669 cm-1 respectively whereas Cu(NO3)2-TGBA (a) and CuCl2-TGIA (a) display only one band at ca.1650 cm-1 of symmetric and antisymmetric bending vibration. Owing to absence of any band characteristic of coordinated water, presence of water in coordination zone of copper (II) is titally refruted.
Magnetic moments of the complexes (1.78-2.18BM) close to spin-only value (1.73B.M), correspond to d9 Cu(II) paramagnetics.Electronic spectra of complexes display four or five bands in 18868-27778 cm-1 , 27778-30769 cm-1 , 30303- 31746cm-1 , 33898- 40000cm-1 and 47619-48780 cm-1 . The first three bands are characteristic of 2B1g → 2A1g , 2B1g → 2B2g and 2B1g → 2Eg d-d transitions of square planar geometry whereas other two bands of high energy could be due to ligand → metal charge transfer.
Based on aforesaid studies following structures of the complexes are proposed (Fig., 2 A &B)
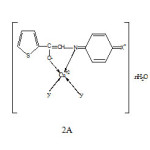 |
Fig2A: Structure of low Rf (a) complexes: Click here to View Figure |
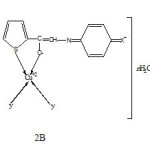 |
Fig2B: Structure of high Rf (b) complexes Click here to View Figure |
Where X= Cl, Br or I and Y= Cl– or NO3– n=0,1 or 3
Antimicrobial studies
Fungicidal data (Table-2) clearly reveals that all the complexes and TGCA are totally non- toxic against both test fungi whereas TGBA and TGIA exhibit dose dependant antifungal activity; TGBA showing better activity than TGIA, almost similar to Bavistin (standard fungicidal drug), could be proposed for further investigation to look at the possibility of its use as selective antifungal drug. Antibacterial data however reveals that all the test compounds except Cu(TGBA)Cl2.3H2O (b) and Cu(TGIA)(NO3)2 (b) are toxic against both bacteria tested all the a-isomers involving NO donor sides of ligands are better than b-isomers involving SO coordination sides of ligands in bactericidal properties. Higher inhibition levels of complexes than their free ligands, observed in majority cases in all the three doses applied, could be attributed to the better lipophilic nature of complexes than ligands on the basis of chelation theory [20].
 |
Table2: Antimicrobial activity of synthesized compounds Click here to View table |
REFERENCES
- Upadhyay RK, Bansal RR, Thin layer chromatographic studies of metal ions complexed with anils I, J. Indian Chem. Soc., 53, 1976, 15.
- Upadhyay RK, Tiwari AP, Analytical application of diethyl aminoanil of phenylglyoxal as gravimetric reagent I; estimation of Hg (II), 57, 1980, 292.
- Upadhyay RK, Agarwal N, Gupta N, Synthesis of antibacterial non-toxic 1,4-thiazolidines, J. Indian Cham. Soc., 70, 1993, 537.
- Vats V, Upadhyay RK, Sharma P, Synthesis and antifungal activity of 2- ketophenyl-3-substituted aryl-1-thiazolidin-4-ones, E-J. Chem.,73, 2010, 1040.
- Gebretekle D, Tadesse A, Upadhyay RK, Dekebo A, Synthesis, characterization and antimicrobial evaluation of some Schiff’s bases and their thiazolidine derivatives, Orient. J. Chem., 28, 2012, 1791.
- Upadhyay RK, Bajpai AK, Rathore K, Chemistry of phenyl glyoxal-p-dimethylaminoanil and /or thiourea substituted ammine complexes, Transition Met. Chem., 10, 1985, 24.
- Upadhyay RK, Chemistry of mixed ligand complexes of Cu (II) involving ketoanil and thiourea/ ammonia/anionic ligands, J. Indian Chem. Soc., 75, 1997, 535.
- Upadhyay RK, Sharma V, Singh VP, Chromatograhic separation of some inorganic isomers, J. Liq. Chromatogr., 5, 1982, 1141.
- Upadhyay RK, Rani A, Thin layer chromatography of binary mixtures of metal complexes including isomers, Chromatographia, 23, 1987, 86.
- Samadhiya S, Halve A, Synthetic utility of Schiff’s bases as potential herbicidal agents, Orient. J. Chem., 17, 2001, 119.
- Upadhyay RK, Agarwal N, Synthesis of some o- hydroxyl phenylglyoxalaryl anils as dyes, Nat. Acad. Sci. Lett., 14, 1991, 251.
- Wei Y, Zhang, Bang-Le, Liu P, He W, Zhang S, The application of chiral Schiff’s base in asymmetric catalysis, Mini reviews in Organic Chemistry, 8, 2011, 1.
- Gitanjali B, Adhithan C, Status report of Chemotherapeutic agents, Indian J. Pharma., 32. 2000, 533.
- Kumari V, Studies on biligand complexes of some 3d block elements, Ph.D. Thesis, C. C. S. University, Meerut, 1982,
- Redha IH, Bayati AL, Mahdi FR, Ahmed AH, Synthesis, spectroscopic and antimicrobial studies of transition metal complexes of N-amino quinolone derivatives, ECSOS, 14, 2010, 1-30.
- Upadhyay RK, Chemistry of ketoanils and their complexes including isomers, D. Sc., Thesis, C. C. S. University, Meerut, 1994.
- Upadhyay RK, Studies of some metal chelates of ketoanils IV, J. Indian Chem. Soc., 72, 1995, 589.
- Upadhyay RK, TLC of Pt-metals complexol with ketoanils: Analysis and effects of chelate ring contraction on Rf values, Chromatographia, 25, 1988,324.
- Lewis J, Wilkins RG, Modern coordination chemistry, Interscience, New Yark, 1964.
- Kumar A, Kumar D, Kumar G, Singh CP, Rana VB, Synthesis, physical characterization and antimicrobial activity of trivalent metal Schiff’s base complexes, J. Serb. Chem. Soc., 75, 2010, 629.

This work is licensed under a Creative Commons Attribution 4.0 International License.









