A Facile Approach of Diels-Alder Reaction in Imidazolium-based Ionic Liquids at Room Temperature
Nur Liyana Sakinah Johari Nur Hasyareeda Hassan* Nurul Izzaty Hassan
1School of Chemical Sciences & Food Technology, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor Darul Ehsan, Malaysia
DOI : http://dx.doi.org/10.13005/ojc/300333
Article Received on :
Article Accepted on :
Article Published : 24 Sep 2014
A Diels-Alder reaction between anthracene and 1-p-tolyl-2,5-dione was conducted in imidazolium-based ionic liquids. Imidazolium cation was utilised with counter anions, [BF4] and [PF6], as the solvents to carry out the desired Diels-Alder reaction. In this work, the diene and dienophile were introduced into the ionic liquids for 72 hours at room temperature. The desired cycloadduct was prepared by a green chemistry procedure in high yield. The expected cycloadduct was characterized on the MS as well as FTIR and NMR spectroscopy. Herein we report, only the [Bmim][BF4] ionic liquid gave the desired cycloadduct in 86 % yield compared to [Bmim][PF6].
KEYWORDS:Diels-Alder reaction; anthracene; imidazolium-based ionic liquids
Download this article as:| Copy the following to cite this article: Johari, N. L. S. Hassan N. H, Hassan N. I. A Facile Approach of Diels-Alder Reaction in Imidazolium-based Ionic Liquids at Room Temperature. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Johari, N. L. S. Hassan N. H, Hassan N. I. A Facile Approach of Diels-Alder Reaction in Imidazolium-based Ionic Liquids at Room Temperature. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=5000 |
INTRODUCTION
Recently, there has been increased attention on the use of ionic liquids as an alternative medium in organic reactions such as the Friedel-Crafts reaction, isomerization and hydrogenation 1-3. In addition, the Diels-Alder reaction performed in ionic liquids has attracted growing interest to meet the awareness of green and environmentally friendly synthesis 4-7. More recently works on the Diels-Alder reaction in imidazolium-based ionic liquids has been well documented 8-14. The Diels-Alder reaction is a pericyclic reaction, and provides cycloadducts as a mixture of isomers, a process which is not always selective 15. It is well known that the selectivity and reactivity of the Diels-Alder reaction are strongly influenced by solvent effects. Consequently, the Diels-Alder reaction has been investigated in various mediums such as water, surfactants, lithium amides, borane-THF complexes and others. Due to that, rate enhancements also have been explored in these solvents 16-19.
As mentioned from previously reported work, ionic liquids have great potential to influence the cycloadducts of the Diels-Alder reaction. In addition, ionic liquids also promote the efficiency of the catalyst in terms of recyclability, as well as being a catalyst itself in the Diels-Alder reaction 20, 21. The use of ionic liquids in the Diels-Alder reaction was first reported by Jaeger and Tucker in 1989. They used ethylammonium nitrate as the solvent in the Diels-Alder reaction between cyclopentadiene and methyl acrylate. Their work successfully yielded a 6.7:1 mixture of endo and exo isomers. The same reaction was conducted in various ionic liquids, including [emim][BF4], [emim][PF6], [emim][NO3], [emim][ClO4] and [emim][CF3SO3] 22-24.
We herein report the synthesis of the imidazolium-based ionic liquids, 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4] and 1-butyl-3-methylimidazolium hexafluorophosphate [Bmim][PF6], and their application as solvents in the Diels-Alder reaction between anthracene and 1-p-tolyl-2,5-dione. These imidazolium-based ionic liquids are purposely replacing molecular solvents in Diels-Alder reaction. There are some previous reports that work on the Diels-Alder reaction by applying other imidazolium-based ionic liquids as solvent and using maleimide as the dienophile 25, 26. However, we believe that this cycloadduct that formed by cycloaddition of anthracene and 1-p-tolyl-2,5-dione was the first time developed in this imidazolium-based ionic liquids, [Bmim][BF4] and [Bmim][PF6], a novel synthetic method for this cycloadduct.
MATERIALS AND METHOD
All melting points were obtained using a Barnstead Electrothermal 9100 melting point apparatus. IR spectra were recorded as KBr disks on a Perkin Elmer Spectrum GX spectrometer. The 1H NMR spectra were obtained using a Jeol spectrometer operating at 400MHz with TMS as an internal standard. 13C NMR spectra were determined using TMS as an internal standard with a Jeol spectrometer operating at 100 MHz. High resolution MS spectra were measured using a Bruker spectrometer in ESI mode. GCMS spectra were obtained using an Agilent 7890A GC coupled with Agilent 5975C MSD. TLC was carried out using Merck Kieselgel 60 PF254 plates. Column chromatography was performed using Merck Kieselgel 60 (0.063–0.200 mm). All organic solvents used in this work were purchased from Sigma-Aldrich, Acros Organic, and Friendemann Schmidt.
Starting materials. 1-p-tolyl-2,5-dione, [Bmim][BF4], and [Bmim][PF6] were synthesized using previously reported synthetic procedures 27,28. Anthracene is commercially available and was recrystallized prior to use. 2-methylfuran, N-methylmaleimide and [Hmim][BF4] are commercially available from the supplier. All other chemicals such as tetrahydrofuran, dichloromethane, diethyl ether, zinc bromide, HMDS, MgSO4, HCl, activated charcoal, basic alumina and acidic alumina used in this work were commercially available and used with no further purification.
1-p-Tolyl-2, 5-dione
In a two-neck round bottom flask, p-toluidine was dissolved in tetrahydrofuran and mixed with maleic anhydride. Mixture was stirred at room temperature under an inert atmosphere overnight. The resulting precipitate was filtered and used in the next step without further purification. The crude p-maleimide acid was suspended in dry acetonitrile. Zinc bromide and HMDS were added to the solution and the resulting reaction mixture was heated at reflux (90°C) for 2 h. The reaction mixture was cooled down at room temperature and filtered. Water was added and the pH of the solution was adjusted to pH1 using 1M HCl. The solution was washed with ethyl acetate, the layers separated and the organic phase dried (MgSO4) 27. The solution was filtered and concentrated to give a yellow powder. Recrystallization from ethyl acetate gave 1-p-tolyl-2,5-dione 84 % as yellow needles. IR (KBr) 3450.67 (OH), 2967.11 (sp3 CH),1702.41 (C=O) 1041.54 (C-N) cm–1; 1H NMR (400 MHz, acetone-d6) δ7.27 (2H, d, J= 8.08 Hz, ArCH), 7.22 (2H, d, J= 8.44 Hz, ArCH), 7.00 (2H, s, HC=CH), 2.35 (3H, s, CH3); 13C NMR (100 MHz, acetone-d6) δ169.8 (C=O), 137.4 (C=C), 134.4 (quaternary C), 129.5 (aromatic C=C), 129.3 (aromatic C=C), 126.5 (quaternary C), 20.0 (CH3); mp: 150.0–150.5 °C; MS (EI+) m/z 187.1 (M+, 100 %).
1-Butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4]
Tetrafluoroborate acid was added dropwise to a cold aqueous solution of 1-butyl-3-methylimidazolium chloride, [Bmim][Cl] in a two-neck round bottom flask. The reaction mixture was stirred at room temperature for 72 h under an inert atmosphere. The resulting ionic liquid was extracted from the aqueous phase using dichloromethane and the layers separated. The dichloromethane layer was washed using distilled water to the excess unreacted acid and chloride salt. The aqueous layer was tested with litmus paper and silver nitrate whilst the dichloromethane layer was concentrated to give a yellow liquid. The yellow liquid was directly treated with activated charcoal and filtered through basic alumina to give 1-butyl-3-methylimidazolium tetrafluoroborate (28 %) as a colorless liquid 28. IR (KBr) 3565.66 (OH), 1635.22 (C=C), 1064.73 (CN) cm–1; 1H NMR (400 MHz, DMSO-d6) δ9.01 (1H, s, CH-2), 7.70 (1H, s, CH-4), 7.63 (1H, s, CH-5), 4.15 (2H, t, J= 7.32 Hz, NCH2CH2CH2CH3), 3.88 (3H, s, NCH3), 1.76 (2H, m, NCH2CH2CH2CH3), 1.25 (2H, m, NCH2CH2CH2CH3), 0.88 (3H, t, J= 7.32 Hz, NCH2CH2CH2CH3); 13C NMR (100 MHz, DMSO-d6) δ 136.9 (NCN), 124.0 (C-4), 122.7 (C-5), 49.0 (NCH2CH2CH2CH3), 36.1 (NCH3), 31.8 (NCH2CH2CH2CH3), 19.1 (NCH2CH2CH2CH3), 13.6 (NCH2CH2CH2CH3); MS (ESI+) m/z 365.25 [2(C4C1im) + BF4] and 139.12 [C4C1im]; MS (ESI-) m/z 313.12 [2(BF4) + C4C1im].
1-Butyl-3-methylimidazolium hexafluorophosphate [Bmim][PF6]
Lithium hexafluorophosphate was mixed with 1-butyl-3-methylimidazolium chloride, [Bmim][Cl] in a two-neck round bottom flask. The reaction mixture was stirred in dry dichloromethane at room temperature for 72 h under an inert atmosphere. The reaction mixture was filtered and the excess lithium chloride was washed with dichloromethane. The combined organic extraction was washed with distilled water until the aqueous layer was free from halide, determined using the silver nitrate test. The dichloromethane layer was concentrated to give a yellow liquid. The yellow liquid was treated with activated charcoal and filtered through acidic alumina to give 1-butyl-3-methylimidazolium hexafluorophosphate (27%) as a colorless liquid 28. IR (KBr) 3392.27 (OH), 1649.54 (C=C), 1008.56 (CN) cm–1; 1H NMR (400 MHz, DMSO-d6) δ 9.00 (1H, s, CH-2), 7.67 (1H, s, CH-4), 7.61 (1H, s, CH-5), 4.14 (2H, t, J= 7.32 Hz, NCH2CH2CH2CH3), 3.83 (3H, s, NCH3), 1.76 (2H, m, NCH2CH2CH2CH3), 1.26 (2H, m, NCH2CH2CH2CH3), 0.89 (3H, t, J= 7.32 Hz, NCH2CH2CH2CH3); 13C NMR (100 MHz, DMSO-d6) δ 136.9 (NCN), 124.0 (C-4), 122.6 (C-5), 49.1 (NCH2CH2CH2CH3), 36.1 (NCH3), 31.8 (NCH2CH2CH2CH3), 19.2 (NCH2CH2CH2CH3), 13.5 (NCH2CH2CH2CH3); MS (ESI+) m/z 423.21 [2(C4C1im) + PF6] and 139.12, [C4C1im], MS (ESI-) m/z 429.05 [2(PF6) + (C4C1im)] and 144.96 [PF6].
Procedure for the preparation of cycloadducts 1 and 2
Reaction between 1-p-tolyl-2,5-dione and anthracene in [Bmim][BF4] at rt. In a two-neck round bottom flask, anthracene and 1-p-tolyl-2,5-dione were added directly into [Bmim][BF4] and the resulting reaction mixture was stirred at room temperature for 72 h under an inert atmosphere. The desired product was removed from the ionic liquid by extracting the solution with diethyl ether. The diethyl ether was removed under vacuum and the residual powder obtained was purified by column chromatography using a mixture of hexane: ethyl acetate (9:1) as the eluent to give the cycloadduct 1 (86%) as a colorless powder. IR (KBr) 3422.16 (OH), 2928.43 (sp3 CH),1650.51 (C=O), 1045.41 (C-N)cm–1; 1H NMR (400 MHz, CDCl3) δ 7.41 (2H, d, J= 8.40 Hz, ArCH), 7.33 (2H, d, J= 8.80 Hz, ArCH), 7.19 (4H, m, ArCH), 7.08 (2H, d, J= 8.04 Hz, ArCH), 6.36 (2H, d, J= 8.08 Hz, ArCH), 4.87 (2H, s, sp CH), 3.36 (2H, s, sp CH), 2.27 (3H, s, CH3); 13C NMR (100 MHz, CDCl3) δ 176.3 (C=O), 141.3 (aromatic C=C), 138.8 (aromatic C=C), 136.9 (aromatic C=C), 129.8 (aromatic C=C), 128.8 (aromatic C=C), 127.2 (aromatic C=C), 126.9 (aromatic C=C), 126.2 (aromatic C=C), 125.2 (aromatic C=C), 124.4 (aromatic C=C), 123.4 (aromatic C=C), 121.8 (aromatic C=C), 50.0 (quaternary C), 47.1 (quaternary C), 45.9 (quaternary C), 36.5 (quaternary C), 31.9 (quaternary C), 29.7 (quaternary C), 28.9 (HC=C), 19.4 (HC=C), 13.4 (CH3); mp 270.0- 270.2°C; MS (EI+) m/z 365.4 (M+, 100%).
Reaction between 1-p-tolyl-2,5-dione and anthracene in [Bmim][BF4] at reflux (90 °C)
In a two-neck round bottom flask, anthracene and 1-p-tolyl-2,5-dione were added directly into [Bmim][BF4] and the resulting reaction mixture was heated at refluxed (90 °C) for 2 h under an inert atmosphere. The desired product was removed from the ionic liquid by extracting the solution with diethyl ether. The diethyl ether was removed under vacuum and residual powder obtained was purified by column chromatography with mixture of hexane: ethyl acetate (9:1) as the eluent to give the cycloadduct 2 (51%) as a colorless powder; IR (KBr) 3421.90 (OH), 2928.43 (sp3 CH), 1685.02 (C=O), 1010.62 (C-N)cm–1; 1H NMR (400 MHz, CDCl3) δ 7.41 (2H, d, J= 8.80 Hz, ArCH), 7.33 (2H, d, J= 8.80 Hz, ArCH), 7.19 (4H, m, ArCH), 7.08 (2H, d, J= 8.40 Hz, ArCH), 6.36 (2H, d, J= 8.44 Hz, ArCH), 4.87 (2H, s, sp CH), 3.36 (2H, s, sp CH), 2.27 (3H, s, CH3); 13C NMR (100 MHz; CDCl3) δ176.3 (C=O), 141.3 (aromatic C=C), 138.8 (aromatic C=C), 137.2 (aromatic C=C), 129.8 (aromatic C=C), 128.7 (aromatic C=C), 127.2 (aromatic C=C), 126.9 (aromatic C=C), 126.2 (aromatic C=C), 125.2 (aromatic C=C), 124.4 (aromatic C=C), 123.3 (aromatic C=C), 121.7 (aromatic C=C), 50.0 (quaternary C), 47.1 (quaternary C), 45.9 (quaternary C), 36.5 (quaternary C), 31.9 (quaternary C), 29.7 (quaternary C), 21.2 (HC=C), 19.4 (HC=C), 13.4 (CH3); mp 270.0- 270.2 °C; MS (EI+) m/z 365.4 (M+, 100%).
RESULTS AND DISCUSSION
Two ionic liquids were synthesized using two synthetic approaches involving acid-based and metathesis methods. [Bmim][BF4] was prepared via an acid-based method whilst a metathesis method was used to synthesize [Bmim][PF6]. In order to get pure ionic liquids, all of the ionic liquids were treated using activated charcoal to remove residual organic materials from the earlier steps of their synthesis. For ionic liquids prepared via the acid-based method, they must be filtered through basic alumina in order to remove the residual acidic materials from the tetrafluoroboric acid reagent. Whilst for the metathesis method, the ionic liquids must be filtered through acidic alumina to remove the residual imidazole starting material.
These two ionic liquids were applied in the Diels-Alder reaction because of the ability of the imidazolium cation to act as a hydrogen bond donor, whilst the BF4 and PF6 counter anions can act as hydrogen bond acceptors. It is well known that Lewis acid catalysts significantly influence the selectivity and reaction rates of the Diels-Alder reaction; the formation of a hydrogen bond between the imidazolium ionic liquid and the 1-p-tolyl-2,5-dione substrate is known as the Lewis acid-base interaction.
Scheme 1 Schematic diagram for the reaction of anthracene and 1-p-tolyl-2,5-dione in ionic liquids.
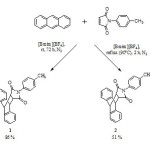 |
Figure 1 Click here to View Figure |
The Diels-Alder reaction between anthracene and 1-p-tolyl-2,5-dione was carried out in the ionic liquids, [Bmim][BF4] and [Bmim][PF6]. Unfortunately, when using [Bmim][PF6] as the reaction solvent, no cycloadducts were observed. Theoretically, the ionic liquid [Bmim][PF6] gave higher yields compared to [Bmim][BF4] due to the lower basicity of PF6. Therefore, further investigation into why no cycloadducts were formed in [Bmim][PF6] is warranted. In contrast, the Diels-Alder reaction carried out at room temperature using [Bmim][BF4] as the reaction solvent gives the cycloadducts in 86% yield, whilst conducting the reaction at reflux gives the cycloadducts in a lower 51% yield. The ability of [Bmim][BF4] to act as a solvent in this reaction was proved and better yields were obtained at room temperature. We believe that this Diels-Alder reaction with the use of catalyst will provide better yields and we are looking forward in developing this reaction.
CONCLUSION
We have shown that Diels-Alder reaction between anthracene and 1-p-tolyl-2,5-dione in [Bmim][BF4] was best in room temperature condition with 86% yield. The ionic liquids that used in this reaction can be potentially recycled after purification process up to five times.
ACKNOWLEDGEMENT
We would like to thank the School of Chemical Sciences and Food Technology, Faculty of Science and Technology and Centre for Research and Instrument (CRIM), University Kebangsaan Malaysia for instrumental facilities as well as grants GUP-2012-073 and GGPM-2013-038 for funding the project and the Ministry of Higher Education.
REFERENCES
- Aggarwal, A.; Lancaster, N. L.; Sethi, A. R.; Welton, T. Green Chem. 2002, 4, 517.
- Behr, A., Naendrup, F.; Nave, S. Eng in Life Sci, 2003, 3, 325.
- Brown, P.; Butts, C. P.; Eastoe, J.; Fermin, D.; Grillo, I.; Lee, H.-C.; Parker, D. Langmuir : the ACS journal of surfaces and colloids. 2012, 28, 2502.
- Burrell, A. K.; Sesto, R. E.; Del, Baker, S. N.; McCleskey, T. M. & Baker, G. A. Green Chem. 2007.
- Doherty, S.; Goodrich, P.; Hardacre, C.; Luo, H.-K., Rooney, D. W.; Seddon, K. R.; Styring, P. Green Chem. 2004, 6, 63.
- Earle, M. J.; Mccormac, P. B.; Seddon, K. R. Green Chem. 1999, 1, 23.
- Fischer, T.; Sethi, A.; Welton, T.; Woolf, J. Tetrahedron, 1999, 40, 793.
- Janus, E.; Goc-Maciejewska, I.; Łozyński, M.; Pernak, J. Tetrahedron Lett. 2006, 47, 4079.
- Meracz, I.; Oh, T. Tetrahedron Lett. 2003, 44, 6465.
- Mirgane, N. A.; Kotwal, S. B.; Karnik, A. V. Central European Journal of Chem. 2010.
- Sun, I.-W.; Wu, S.-Y.; Su, C.-H.; Shu, Y.-L.; Wu, P. L. Journal of the Chinese Chemical Society (Taipei, Taiwan). 2004, 51, 367.
- Xiao, Y.; Malhotra, S. V. Tetrahedron Lett. 2004, 45, 8339.
- Yadav, J. S.; Reddy, B. V. S.; Reddy, J. S. S.; Rao, R. S. Tetrahedron. 2003, 59, 1599.
- Stefaniak, W.; Janus, E.; Milchert, E. Cataly Lett. 2011, 141, 742.
- Shen, Z. L.; Sheong, H. L.; Lai, W. C.; Loo, W. Y.; Loh, T. P. Green Chem. 2012, 14, 2626.
- Song, C. E.; Shim, W. H.; Roh, E. J.; Lee, S.; Choi, J. H. Chem Comm. 2001, 24, 1122.
- Kumar, A.; Pawar, S. S. J. Org. Chem. 2007, 72, 8111.
- Takahashi, K.; Nakano, H.; Fujita, R. Chem. Comm. 2007, 263.
- Bini, R.; Chiappe, C.; Mestre, V. R.; Pomelli, C. S.; Welton, T. Org. Biomol. Chem. 2008, 6, 2522.
- Welton, T. Chem Rev. 1999, 99, 2071.
- Gordon, C. M. App Catalysis A: General. 2001, 222, 101.
- Welton, T. Coord. Chem Rev. 2004, 248, 2459.
- Keskin, S.; Talay, D. K.; Akman, A.; Hortacsu, O. J. Supercrit. Fluids. 2007, 43, 150.
- Bourbigou, H. O.; Magna, L.; Morvan, D. App. Catalysis A: General. 2010, 373, 1.
- Reddy, P. Y.; Kondo, S.; Fujita, S.; Toru, T. Synthesis. 1998, 7, 999.
- Reddy, P. Y.; Kondo, S.; Toru,; Ueno, Y. J. Org. Chem. 1998, 7, 999.
- Hassan, N.I. Ph.D. University of Saint Andrew, United Kingdom, 2012.
- Hassan, N.H. Ph.D. Imperial College London, United Kingdom, 2012.

This work is licensed under a Creative Commons Attribution 4.0 International License.









