Zn(BH4)2/Ultrasonic Irradiation: An Efficient System for Reduction of Carbonyl Compounds to their Corresponding Alcohols
Siamak Fanari and Davood Setamdideh*
Department of Chemistry, College of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, Iran,
DOI : http://dx.doi.org/10.13005/ojc/300240
Article Received on :
Article Accepted on :
Article Published : 28 Jun 2014
Zn(BH4)2 under ultrasonic irradiation is an efficient reducing system in CH3CN. This system reduces a variety of carbonyl compounds to their corresponding alcohols at room temperature in high to excellent yields of the products. Also, a,b-unsaturated aldehydes and ketones was regioselectively reduced to the corresponding allylic alcohols.
KEYWORDS:Zn(BH4)2; Ultrasonic Irradiation; Carbonyl Compounds; Alcohols
Download this article as:| Copy the following to cite this article: Fanari S, Setamdideh D. Zn(BH4)2/Ultrasonic Irradiation: An Efficient System for Reduction of Carbonyl Compounds to their Corresponding Alcohols. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Fanari S, Setamdideh D. Zn(BH4)2/Ultrasonic Irradiation: An Efficient System for Reduction of Carbonyl Compounds to their Corresponding Alcohols. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=4005 |
Introduction
Zn(BH4)2 is a non-conventional hydride transferring agent which has been reported as an efficient chemo-, regio- and stereoselective reducing agent in several complex substrates. It is moderately stable in ethereal solution which can be used in a range of aprotic solvents such as, THF, Et2O and DME. Several Combination reducing systems of Zn(BH4)2 such as Zn(BH4)2/TMEDA 1, Zn(BH4)2/Me3SiCl 2, Zn(BH4)2/TFA/DME 3, Zn(BH4)2/H2O 4, Zn(BH4)2/Al2O3 5, Zn(BH4)2/C 6, Zn(BH4)2/ZrCl4 7 and Zn(BH4)2/2NaCl 8 have been used for the different reduction purposes. Also, it is well known that sonication as unconventional energy is a well tool to facilities for varieties of synthetic methods on organic chemistry 9-10. In this context we wish to introduce Zn(BH4)2 under ultrasonic irradiation as a new combination reducing system for convenient reduction of a variety of carbonyl compounds such as aldehydes, ketones and α,β-unsaturated carbonyl compounds to their corresponding alcohols at room temperature.
Results and Discussions
We have chosen the reduction of benzaldehyde as a model in order to determine the appropriate reaction conditions and evaluate the efficiency of ultrasonic irradiation.The reduction of benzaldehyde took place with one molar equivalent of Zn(BH4)2 in THF within 30 minutes at -10 °C as shown in scheme 1. Among the tested aprotic and protic solvents i.e. n-hexane, CHCl3, CH2Cl2, Et2O, DME, CH3CN, THF, CH3OH, C2H5OH and solvent-free conditions the reduction of benzaldehyde was better in CH3CN. The optimization study showed that using 1 molar equivalents of Zn(BH4)2 in CH3CN (3 mL) is the best conditions. Our observation reveals that reduction reaction completes within 5 min at room temperature with 95% yields of product as shown in scheme 1.
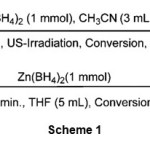 |
Scheme 1 |
This procedure was also applied for the reduction of various aldehydes to the corresponding primary alcohols (Table 1, entries 1-7). All reductions were completed within 5-10 min by 1 molar equivalents of Zn(BH4)2 under ultrasonic irradiation with excellent yields of the products (91-96%).
 |
Table1: Reduction of Carbonyl Compounds by Zn(BH4)2 under Ultrasonic Irradiation in CH3CN. Click here to View table |
Our next attempt was the reduction of ketones to the corresponding secondary alcohols. The reduction of ketones was also obtained successfully by 2 molar equivalents of Zn(BH4)2 under ultrasonic irradiation within 60-120 min at room temperature in CH3CN with excellent yields of the products (90-94%) (Table 1, entries 8-14).
Also, we have examined the reduction of cinnamaldehyde (Table 1, entry 7) and benzalacetone (Table 1, entry 11) as models for a,b-unsaturated aldehydes and ketones. The reduction reactions took place to their corresponding allylic alcohols in excellent yields in CH3CN at room temperature as shown in scheme 2.
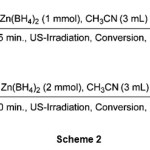 |
Scheme 2 Click here to View Scheme |
Experimental
Sonication was performed by using a Bendeline uw 3100 (Germany) high intensity ultrasonic (600 W, 20 KHz) via a micro-tip probe (vs 70t) and 70% amplitude. IR and 1H NMR spectra were recorded on Perkin-Elmer FT-IR RXI and 300 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples. TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
Typical procedure for the reduction of carbonyl compounds with Zn(BH4)2 under ultrasonic irradiation in CH3CN
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer bar, a solution of benzaldehyde (0.1061 g, l mmol) was prepared in CH3CN (3 mL). To this solution Zn(BH4)2 (0.095 g, 1mmol) was added. The resulting mixture was stirred under ultrasonic waves at room temperature for 5 min. The progress of the reduction reaction was monitored by TLC (eluent:CCl4/Et2O:5/2). After completion of the reaction, distilled water (5 mL) was added to the reaction mixture and stirred for 5 min. The mixture was extracted with CH2Cl2 (3×10 mL) and dried over anhydrous Na2SO4. Evaporation of the solvent afforded pure benzyl alcohol (0.102 g, 95% yield).
Conclusion
In this context, we have shown that Zn(BH4)2 under ultrasonic irradiation as new reducing system is convenient for the reduction of aldehydes and ketones to their corresponding alcohols. Also, a, b-unsaturated aldehydes and ketones are regioselectively reduced to the corresponding allylic alcohols. The reduction reactions were carried out with Zn(BH4)2 (1-2mmol) in CH3CN at room temperature. Short reaction times and easy work-up procedure makes as an attractive new protocol for the reduction of carbonyl compoundsto their corresponding alcohols.
Acknowledgements
The authors gratefully appreciated the financial support of this work by the research council of Islamic Azad University branch of Mahabad.
References
- Kotsuki, H.; Ushio, Y.; Yoshimura, N.; Ochi, M. Bull. Chem. Soc. Jpn. 61: 2684 (1988).
- Kotsuki, H.; Ushio, Y.; Yoshimura, N.; Ochi, M. J. Org. Chem. 52: 2594 (1987).
- Ranu, B. C.; Das, A. R.J. Chem. Soc. Perkin Trans. 1 1561 (1992).
- Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M.; Aliporamjad, A. Asian J. Chem. 24: 3591(2012).
- Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M. J. Serb. Chem. Soc. 79: 1 (2013).
- Setamdideh, D.; Rahmatollahzadeh, M. J. Mex. Chem. Soc. 56: 169 (2012).
- Kamari, R.; Setamdideh, D. Orient. J. Chem. 29: 497 (2013).
- Setamdideh, D.; Khaledi, L. S. Afr. J. Chem. 66: 150 (2013).
- Mason, T. J.; Lorimer, J. P. applied sonochemistry: uses of power ultrasonic in chemistry, John Wiley & sons (2003)
- Mason, T. J. Sonochemistry, Oxford Univesirty Press, Oxford (1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









