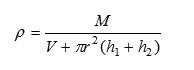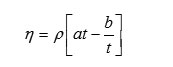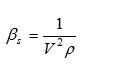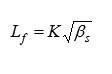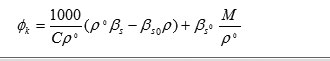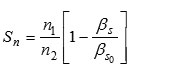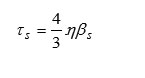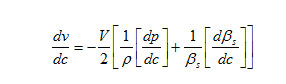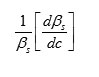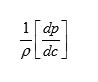The Physico-Chemical and Solvolytic Study of Alprazolam Drug In Ethanol at 303 K
Chandra Kant Bhardwaj1*, Anjna Kumari2, Anjul Singh2
*1Department of Chemistry, Doon Institute of Engg. and Technology, Rishikesh, U.K.(India) 2Department of Chemistry D. S. College, Aligarh, U.P. [India]
DOI : http://dx.doi.org/10.13005/ojc/300261
Article Received on :
Article Accepted on :
Article Published : 04 Jun 2014
The physico-chemical and solute –solvent interaction of Alprazolam in ethanol were reported at 303 K. The solute solvent interaction have been carriedout by computing various acoustic parameters, Specific acoustic impedance(z),Intermolecular freelength(Lf),Isentropic compressibility (bs) , Apparent molal adiabatic compressibility (fk ) , Shear’s relaxation ( ts ), and Solvation number (Sn).These parameters have been evaluated by using ultrasound velocity, density and viscosity data.These results are interpreted in terms of solute-solvent interaction between the molecules.
KEYWORDS:Solute-solvent interaction; Ultrasound velocity; Alprazolam.
Download this article as:| Copy the following to cite this article: Bhardwaj C. K, Kumari A, Singh A. The Physico-Chemical and Solvolytic Study of Alprazolam Drug In Ethanol at 303 K. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Bhardwaj C. K, Kumari A, Singh A. The Physico-Chemical and Solvolytic Study of Alprazolam Drug In Ethanol at 303 K. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3554 |
Introduction
Alprazolam is a triazolobenzodiazepine compound with antianxiety and sedative-hypnotic actions, that is efficacious in the treatment of panic disorders, with or without agoraphobia and in generalized anxiety disorders.It is marketed under the trade name xanax.
In the present study, we are reported the ultrasound velocity, density and viscosity measurements at 303K have been used to calculate Intermolecular freelength(Lf) [1] , Specific acoustic impedance(z) [2], Apparent molal adiabatic compressibility (fk ) [3] , Shear’s relaxation ( ts ), and Solvation number (Sn) [4] of Alprazolam compound in ethanol at 303 K . Several investigators have reported the results on ultrasound studies of liquid mixtures [4-12].
EXPERIMENTAL
The solutions were prepared by dissolving the accurately known weight of Alprazolam in ethanol and kept for some time. A continuous interferometer technique was employed for measurement of ultrasound velocity at 2Mhz. The density and viscosity were determined using vibrating densitometer DMA 48 fitted with a Hook G thermostat and viscometer. The experiment were reported atleast twice and results were reproducible with experimental error 0.00002 Kgm3 and 0.001 cm/ sec respectively.
Computation of different physical parameters
Ultrasound Velocity (V)
V = 2d × 105 cm/ sec. or V = 2d × 103 m/ sec
Density
where ‘M’: is the weight of the liquid filled in pyknometer used.
‘r’is radius of capillaries
‘h1’& ‘h2’are the heights of the liquid in capillaries
Viscosity ( η)
where
‘h’ is the viscosity of liquid
‘r’ is the density of liquid.
‘t’ is the time flow of liquid
‘a’&’b’ are viscometeric constants.
Specific Acoustic Impedance
Z = Vr.
Where, V and ‘p’ are the ultrasonic velocity and density respectively.
Isentropic Compressibility
where ‘V’ is the ultrasound velocity and ‘p’ is the density of liquid mixtures.
Intermolecular Free Length
where,
‘K’ is temperature dependent constant.
Molal Adiabatic Compressibility
where ‘p0’ and ‘βs0’ are compressibility and density of pure solvent and ‘βs’ & ‘p’ are the compressibility and density of the solution respectively.
‘C’ is the concentration in mole/liter of solute.
‘M’ is the molecular weight of solute.
Solvation Number
Where ‘ n1 ‘ moles of solvent and ‘ n2 ‘ moles of solute
Shear’s Relaxation Time (t)
RESULT AND DISCUSSION
The ultrasound velocity of the solution of Alprazolam drug in ethanol increases with increasing concentration Alprazolam drug which is shown in above table.
Table-: Alpra zolam + ethanol At 303K
|
Conc. of ethanol mol/litre |
Ultrasound velocity m/sec. |
Density gm/mol. (Exp.) |
Specific Acoustic Impedance (C.G.S.) z´10-5 |
Isentropic compressibility (Exp.) cm2/dyne.1012 |
Lowering Isentropic compressibility cm2/deyne.1012 |
Intermolecular free length (Ao) |
Viscosity (Exp.) (C.P.) |
Apparent molal Adiabatic compressibility cm2/dyne.109 |
Solvation number |
Shear’s Relaxation time |
|
0.0025 |
1166 |
0.7883 |
0.0919 |
93.31 |
-6.76 |
0.6095 |
1.2546 |
-274.1037 |
46.2196 |
156.0890 |
|
0.0050 |
1170 |
0.7923 |
0.0927 |
92.20 |
-7.87 |
0.6059 |
1.2960 |
-167.4630 |
53.8089 |
159.3216 |
|
0.0075 |
1175 |
0.7965 |
0.0936 |
90.94 |
-9.13 |
0.6017 |
1.3374 |
-134.2552 |
62.4238 |
162.1642 |
|
0.0100 |
1178 |
0.8010 |
0.0944 |
89.97 |
-10.10 |
0.5985 |
1.3786 |
-115.1329 |
69.0559 |
165.3769 |
|
0.0125 |
1184 |
0.8052 |
0.0953 |
88.59 |
-11.48 |
0.5939 |
1.4201 |
-106.6343 |
78.4912 |
167.7422 |
|
0.0150 |
1188 |
0.8091 |
0.0961 |
87.57 |
-12.50 |
0.5905 |
1.4515 |
-98.3142 |
85.4652 |
169.4771 |
|
0.0175 |
1190 |
0.8137 |
0.0968 |
86.78 |
-13.29 |
0.5878 |
1.5026 |
-91.5657 |
90.8666 |
173.8608 |
|
0.0200 |
1196 |
0.8178 |
0.0978 |
85.49 |
-14.59 |
0.5834 |
1.5442 |
-88.7363 |
99.6866 |
176.0182 |
|
0.0225 |
1199 |
0.8199 |
0.0983 |
84.84 |
-15.23 |
0.5812 |
1.5853 |
-82.4719 |
104.1308 |
179.3291 |
|
0.0250 |
1202 |
0.8263 |
0.0993 |
83.76 |
-16.31 |
0.5775 |
1.6271 |
-81.4079 |
111.5150 |
181.7145 |
The variation of velocity with concentration ( C) can be expressed by the following relationship.
The result shows that while the density increases, the isentropic compressibility decreases with increasing concentration of the solute and quantity (dp/dc) is positive while (dβs/ dc) is negative. Since the value of
are larger than the value of
for the system . The concentration derivatives of velocity( dv/dc) is positive. i.e. the ultrasonic velocity increases with increasing the concentration of solute (13-16).
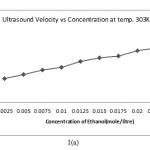 |
Figure 1. A Click here to View Figure |
Intermolecular free length and isentropic compressibility ( βs ) of Alprazolam drug solution decreases with increase in molar concentration of solute (Fig.1-b) .The complementary use of isentropic compressibility data can provide interesting information of of solute- solvent interaction.
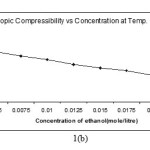 |
Figure 1. B Click here to View Figure |
Apparent molal adiabatic compressibility () varies linearly s the square root of concentration.The value of apparent molal adiabatic compressibilities are negative with the increase in molar concentration. The values of apparent molal adiabatic compressibility as shown in figure 1 (c) . The values of for the solutions of Alprazolam drug were tabulated in table. These results are in agreement with the result reported by Masson (17) for electrolytic solution.
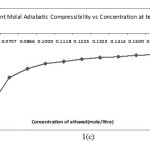 |
Figure 1. C Click here to View Figure |
The value of specific acoustic impedance (z) increases with increasing the concentration of Alprazolam drug can be explained on the basis of lyophobic interaction between solute and solvent molecules which increase the intermolecular distance making relatively under gaps between the molecules and becoming the main cause of impedance in the propagation of ultrasound waves are tabulated.
The increase with the concentration suggest a significant interaction between the solute solvent molecules and the values are in agreement with the reported for solution of cobalt carboxylates (18).
From the above discussion it is concluded that solvolytic study of Alprazolam in ethanol at 303 K shows specific ion – solvent interaction. Alprazolam drug shows significant solvolysis in ethanol.
REFRENCES
- Jacobson,B., Acta Chem.Scand., 6 : 1485 (1952).
- Elpiner,I.E., Ultrasound Physical, Chemical& Biological effects. New York Consultants Bureau, 371 (1960).
- Passynskii, A., Act Physico Chem., (U.S.S.R), 8 : 357 (1933); J. Phys. Chem., (U.S.S.R.), 11: 451 (1938).
- Hori, Mistsukazu, Mishiwki, Nobuhiko, Kata Gijustu, 17(12) :45-48 (2002).
- Kannappan, A.N. and Palani, Rashid, Indian J. Chem., Vol. 46A : 54-59 (2007).
- Josh, M.N., Diego, A.H., J. Chem. Engg. Data, 51 (2) :722 (2006).
- Begum, Zareena, Sandhya Sri, P.B., Karuna Kumar, D.B., Rambabu, C., Journal Of Molecular Liquids, vol. 178 : 99- 112 (2013).
- Prabhabati, C.L., Shiv Kumar, K., Venkatashwarlu, P. and Raman, G.K., Ind. J. Chem., 43A , : 294 (2004).
- Mehra, Rita and Israni, Rekha, Ind. J. Chem. Soc., 81 : 221 (2004).
- Das, U.N., Roy, G.S., and Mohanty, S., Ind. J. of Chem. Tech., 11 : 714 (2004).
- Prakash, S., Chaturvedi,C.V., Ind. J. Chem., 10 : 669 (1972) .
- Waeissler, A., J.Chem. Phys., 15 : 210 (1947).
- Sharma, R.K., Bhardwaj , C.K., Yadav, S.S., Material Science Research India, 3(1a) : 97-102 (2006).
- Ramabrahman, K. and Suryanarayan, M., Ind. J. Pure Appl. Phys., 6, 422 (1968).
- Miknailor, I.G., Rozina, M.V. & Snutilov, V.A., Akust. Zh., 10 : 213 (1964).
- Bachem, C., Physica (Netherlands), 101: 541 (1935).
- Masson, D.O., Phil. Mag. B., 218 (1929).
- Padmini, P. and Rao, B., Indian J. Phys. 34 : 565 (1960)

This work is licensed under a Creative Commons Attribution 4.0 International License.