Synthesis of 5- [(11Substituted Phenothiazinoacetyl)Semicarbazidothio -Semicarbazido]-2- Oxo/Thiobarbiuric Acid as Anticonvulsant Agents
Mirdula Tyagi and Archana
Medicinal Chemistry Lab Department of Chemistry, Meerut College, Meerut – 250004, U.P. (India)
DOI : http://dx.doi.org/10.13005/ojc/300248
Article Received on :
Article Accepted on :
Article Published : 14 Apr 2014
2-Substitutedphenathiazine was prepared according to the reported method. Ethyl-substitutedphenothiazinoacetates(1a-1b) were prepared by the reaction of ethylchloroacetate in the presence of anhydrous K2CO3. 2-amino-5-N10substituted phenothiazinoacetyl) semicarbazdes/thiosemicarbazides (2a-2d) were synthesized by refluxing compound (1a-1b) with thiosemicarbazide/semicarbazide. Compounds (2a-2d) were then underwent mannich reaction to yield compounds 3a-3d. All the newly synthesized compounds (i.e. 2a-2d and 3a-3d) were evaluated for their anticonvulsant activity. Almost all the compounds have shown promising anticonvulsant activity. Compound 3d was the most potent compound of this series. The most potent compound was evaluated for its anticonvulsant activity and acute toxicity.
KEYWORDS:Substitutedphenothiazinoacetylsemicarbazides/thiosemicarbazides, substitutedphenothiazinoacetylsemicarbazido/thiosemicarbazido-2 oxo/thiobarbituric acids, anticonvulsant activity, acute toxicity.
Download this article as:| Copy the following to cite this article: Tyagi M, Archana. Synthesis of 5- [(11Substituted Phenothiazinoacetyl) Semicarbazidothio -Semicarbazido]-2- Oxo/Thiobarbiuric Acid as Anticonvulsant Agents. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Tyagi M, Archana. Synthesis of 5- [(11Substituted Phenothiazinoacetyl) Semicarbazidothio -Semicarbazido]-2- Oxo/Thiobarbiuric Acid as Anticonvulsant Agents. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3025 |
INTRODUCTION
Research in the field of barbituric acid derivatives has yielded a number of clinically useful anticonvulsant drugs. 10H- Phenothiazine nucleus has gained prominance due to its diverse activities like-anticonvulsant,1-6antinflammatory7and cardiovascular8. The introduction of various heterocyclic / aliphatic moieties at 10- position of phenothiazine led to the discovery of promethazine [10-(2-methylaminopropyl) phenothiazine hydrochloride]. Chlorpromazine[2-chloro-10-3-(3-dimethylaminopropyl)phenothiazinehydrochloricle], which posses potent antihistamic and CNS depressant activities respectively. Researchers has revealed that various 5-substituted barbituric acid derivatives possess potent anticonvulsant activity9-16. In view of these observations, it was thought worthwhile to synthesize a new series of 5- substituted barbituric acid derivatives bearing phenothiazinyl moiety. All the newly synthesized compounds were evaluated for their anticonvulsant activity and acute toxicity studies.
Ethylsubstitutedphenothiazinoacetates (1a-1b) were prepared by reacting phenothiazine/substitutedphenothiazine with ethylchloroacetate (in acetone) in the presence of small amount of anhydrous K2 CO3. Appearance of a quartet at d 4.25 due to 2-protons of COOCH2 CH3 group and a triplet at d 1.40 due to 3-protons of COO CH2 CH3 group in the 1H NMR spectrum and a band at 2860 cm-1due to CH2, 3044 cm-1(aromatic C-H), 2960 cm-1 (aliphatic C-H), 1742 cm-1(C=O), 1560 cm-1 (C…C of aromatic ring), 1250 cm-1(C-N) and 1140 cm-1 (C-S) group which were present in the IR spectrum of compound 1a proves the formation of compound 1 a. Compounds (1a-1b) were refluxed with thiosemicarbazide/semicarbazide to yield compounds (2a-2d). The IR spectrum of compound 2c showed additional peaks of 3340 cm-1 (due to NH NH2), 1160 cm-1 (due to C=S) and 1670 cm-1 (due to CO of CO NH). Its 1H NMR spectrum exhibited signals at d 7.72 (m, 4H, NHNH CSNH2), which confirms the structure of compound 2c. Compounds. (2a-2d) were then underwent mannich reaction to yield compounds 3a-3d. The formation of compound 3c is supported by the disappearance of a multiplet at d7.75 due to 4 protons of (NHNHCSNH2) and appearance of a multiplied at 8.30 due to 3- protons of (NHNHCSNH), a single singlet at d 9.25 due to 1-proton of (NHCO), a singlet at d 2.95 due to the 1- proton of CH of barbituric acid and a doublet at 4.45 due to 2-protons of (NHCH2) in the 1H NMR spectrum. Its structure is further confirmed by the presence of bands at 1720, 1710, 1680 due to (C=O of barbituric acid, amidic CO).
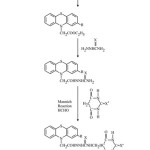 |
Scheme 1: Click here to View Scheme |
Pharmacological activities
Anticonvulsant activity
Maximum electroshock seizure (MES) test
This test was performed according to the method of Tomanet. al17 The group of ten rats was treated with test drugs (50 mg/kg i.p.) / phentoin sodium (30 mg/kg i.p.) after 1h they were subjected to the shock of 150 mA by convulsiometer through ear electrodes for 0.2s and the presence or absence of extensor response was noted. Animals in which extensor response was abolished were taken as protected rats.
Pentylenetetrazole (PTZ) induced seizures Test
This test was performed by following the method of Fischer18. The rats were injected with pentylenetetrazol in dose of 70 mg /kg subcutaneously in scruff of neck. After 2-4 min. of PTZ injection animals developed sequence of excitement, myclonic jerks, clonic seizures, one or more maximum tonic seizures. Animals exhibiting these seizures patterns were selected.Standard drug used in this model was sodium valproate (80 mg/kg i.p.) and was injected 60 min prior to PTZ challenge. All the newly synthesized compounds were studied for their anticonvulsant activity at a dose of 50 mg /kg i.p. in maximal electroshock and pentylenetetrazole induced seizures respectively. All the mewly synthesized compounds have shown anticonvulsant activity in both the models (ranging from 30 to 90% and 20 to 90% in MES and PTZ models, respectively). The anticonvulsant activity of all the compounds are reported in Table 2. Compound 3d was found to possess potent anticonvulsant activity it was studied three graded doses (17. 5, 25, and 50 mg/kg i.p.).
Table 1: Physical and analytical data of compounds (1a-1b), (2a-2d), and (3a-3d)
|
S. No. |
R. |
X |
X1 |
M.P. OC |
Yield % |
Recrystallisation solvent |
Molecular formula (Mol. Wt. ) |
Elemental Analysis % |
|||||
|
C Calc. Found |
H Calc. Found |
N Calc. Found |
|||||||||||
|
1a |
H |
– |
2030C |
80% |
Methanol |
C16H15NO2S (285) |
67.36 |
67.33 |
5.26 |
5.27 |
4.91 |
4.90 |
|
|
1b |
Cl |
– |
2100C |
70% |
Methanol |
C16H14NO2SCl (319.5) |
60.09 |
60.12 |
4.38 |
4.35 |
4.38 |
4.42 |
|
|
2a |
H |
O |
1300C |
75% |
Ethanol/water |
C15H14N4SO2 (314) |
57.32 |
57.36 |
4.45 |
4.42 |
17.83 |
17.87 |
|
|
2b |
Cl |
O |
1000C |
70% |
Methanol/water |
C15H13N4SO2Cl (348.5) |
51.64 |
51.68 |
3.73 |
3.70 |
16.06 |
16.10 |
|
|
2c |
H |
S |
161-1620C |
70% |
Ethanol/water |
C15H14N4S2O (330) |
54.54 |
54.58 |
4.24 |
4.22 |
16.96 |
16.93 |
|
|
2d |
Cl |
S |
1500C |
75% |
Methanol/water |
C15H13N4S2OCl (364.5) |
49.38 |
49.36 |
3.56 |
3.54 |
15.36 |
15.33 |
|
|
3a |
H |
O |
1800C |
80% |
Methanol/water |
C20H18N6O5S (454) |
52.86 |
52.84 |
3.96 |
3.92 |
18.50 |
18.54 |
|
|
3b |
Cl |
O |
1700C |
80% |
Methanol/water |
C20H17N6O4 S2Cl (504.5) |
47.57 |
47.54 |
3.36 |
3.33 |
16.65 |
16.68 |
|
|
3c |
H |
S |
1900C |
70% |
Methanol/water |
C20H18N6O4 S2 (470) |
51.06 |
51.10 |
3.82 |
3.80 |
17.87 |
17.90 |
|
|
3d |
Cl |
S |
1750C |
75% |
Methanol/water |
C20H17N6O3S3Cl (520.5) |
46.10 |
46.08 |
3.26 |
3.29 |
16.13 |
16.17 |
|
Acute toxicity study
ALD50values of some promising compounds were determined by observing 50% mortality after 24 hr19. ALD50value of most active compound i. e. 3d was > 2000 mg/kg p.o. (maximum dose tested).
Experimental
Melting points were taken in open capillary tubes and are uncorrected. Analytical data of C, H, N, were within ±0.04% of the theoretical value. IR spectra (KBr) are recorded on BackmannAcculab -10- spectrophotometer. 1H NMR spectra were recorded by Bruker WM 400 FT instrument using CDCl3as solvent and tetramethylsilance (TMS) as internal reference standard. All chemical shift (d) are in ppm. The purities of the compounds were checked by thin layer chromatography (TLC) on silicagel – G plates of 0.5 mm thickness. The elemental analysis of the compounds were performed on Heracus Carlo Erba 1108 analyser.
Table-2: Pharmacological data of Compounds (2a-2d) and (3a-3d)
|
Comp. |
R |
X |
Dose (mg/kg i.p.) |
Anticonvulsant Activity |
ALD50 |
|
|
MES |
PTZ |
|||||
|
2a |
H |
O |
50 |
30 |
20 |
>1000 |
|
2b |
Cl |
O |
50 |
40 |
30 |
>1000 |
|
2c |
H |
S |
50 |
40 |
40 |
>1000 |
|
2d |
Cl |
S |
50 |
50 |
40 |
>1000 |
|
3a |
H |
O |
50 |
50 |
50 |
>1000 |
|
3b |
Cl |
O |
50 |
60 |
60 |
>1000 |
|
3c |
H |
S |
50 |
70 |
60 |
>1000 |
|
3d |
Cl |
S |
50 25 17.5 |
90 60 30 |
90 50 30 |
>2000 |
|
Phenytoin sodium |
30 |
80 |
|
|||
|
Sodium valproate |
80 |
80 |
|
|||
|
Propylene glycol |
50 |
0 |
0 |
|
||
Preparation of ethylsubstitutedphenothiazinoacetates (1a-1b)
A mixture of substitutedphenothiazine/phenothiazine (0.01 mole), ethylchloroacetate(0.01 mole) and anhydrous K2CO3(8.0 gm) in acetone (90 ml) were refluxed for 24 hours. After refluxing, the excess of solvent was distilled off. The reaction mixture was cooled, filtered and washed with water and recrystallized from appropriate solvents
Preparation of 2-amino-5- (N10 – substitutedphenothiazinoacetyl) semicarbazides/ thiosemicarbazides (2a-2d)
Compounds (1a-1b) (0.075 mole) and thiosemicarbazide / semicarbazide (0.075 mole) in methanol (dry 70 ml) were refluxed on a steam both for about 15 hrs. The excess of the solvent was distilled off and the viscous mass poured into ice – cold water, filtered and recrystallised from appropriate solvents.
Preparation of 5- [(11 – N10– substituted phenothiazinoacetyl) thiosemicarbazido / semicarbazido] -2- oxo/thiobarbituric acids (3a-3d).
Compounds (2a-2d) underwent mannich reaction to yield compounds (3a-3d). To a solution of oxo/thibarbituric acid (0.01 mole) in methanol (50-70 ml), formaldehyde (0.02 mole) and compounds (2a-2d) (0.02 mole) were added dropwise and the reaction mixture was refluxed for 4 hours. The excess of the solvent was distilled off and the solid thus obtained were washed with petrolem ether (40-600C) and recrystallised from appropriate solvents .
REFERENCES
- Hemlata, K., Kumar, S.,Lata S., Saxena, K.K. and Kumar, A., Eur. J. Med. Chem. 46 (2010).
- Srivastava, S.K., Srivastava, S.L. &Srivastava, S.D., J. Indian. Chem. Soc. 77, 104 (2000).
- Scott, K.R., Laws, M.L., Roberts, R.R. and Nicholson, J.M., Chem. Abstr. 130, 311808 d (1999).
- Mishra, R.S., Chaudhary, A., Chaturvedi, A.K., Parmar, S.S. and Shastri, B.V., Chem. Abstr. 88, 58 217 g (1997).
- Helsey, G.C., Davis. L. and Olsen, G.E., Chem. Abstr. 110, 173246c (1989).
- Anfinogenov. V.A., Gorshkova, V.K., Napilkova, O.A., Sartikov. A.S. and Filimonov, V.D., Chem. Abstr. 68739b (1988).
- Mishra, S., Srivastava, S.K. and Srivastava, K., Indian J. Chem. 36 B, 826 (1997).
- Kumar, A., Ram, T., Tyagi, R., Goel, B., Bansal, E. and Srivastava, V.K., Bll. Chem. Farma 137 (5), 152 (1998).
- Siddiqui, N.A. and Ahsan, W., Arch. Pharma. Chem. Life Sci. 342, 173 (2009).
- Goel B., Sharma, S., Bajaj, K., Bansal, E., Singh, T., Malik N., Lata, S., Tyagi, C., Panwar, H., Agarwal, A. and Kumar, A., Indian. J. Pharma Sci. 67, 197 (2005).
- Archana, Rani P., Bajaj K., Srivastava V.K., Chandra R. & Kumar A., Arzneim. Forsch / Drug Res. 53, 301 (2003).
- Archana, Srivastava, V.K. and Kumar, A., Bioorganic and Med. Chem. 1257 (2004).
- Sharma, G.V. S.P. Rao, J.V. and Suresh, B., Chem. Abstr. 133, 120291g (2000).
- Abd El-Hamide, S.G., El- Hakim, A.E., El-Helley, A. A., Chem. Abstr. 126, 22569g (1997).
- Osman A. N., Kandel M.M. & Ahmed M., Indian . J. Chem. 35 B, 1073 (1996).
- Sarma, G.V.S.P., Rao, J.V. and Suresh B., Indian J. Pharma. Sci. 61, 105 (1995).
- Toman J.E.P. Swingarel E.A. & Goodman L.S., Neuro J. Physiol. 231 (1946) .
- Smith Q.E., Medicinal Chemistry, Vol. 1, Butter Worths London (1960).

This work is licensed under a Creative Commons Attribution 4.0 International License.









