QTAIM and NBO Analysis of a New Oxidative Salt Of1,1/-(Ethane-1, 2-Diyl) Dipyridiniumbisiodate
Mohammad Khavani*, Mohammad Izadyar, MostafaGholizadeh, Mohammad Reza Housaindokht
Department of Chemistry, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad, Iran
DOI : http://dx.doi.org/10.13005/ojc/300252
Article Received on :
Article Accepted on :
Article Published : 28 Jun 2014
A theoretical DFT study was employed to investigate the different aspects of electronic structure of the salt of 1,1/(ethane-1,2-diyl)dipyridiniumbisiodate. The interactions between different molecular orbitals were considered by using DFT method. The results from the quantum mechanical calculations were then used to determine the role of donor- acceptor interactions on the oxidation strength of this salt. Theoretical calculations at the B3LYP/LANL2DZ level of the theoryon the molecular entropy and specific heat capacity values over a wide temperature range of 300 to 3000 K confirmed that temperature dependence of vibrational entropy and specific heat capacity is more important than other contributions. Hydrogen bondinteractions were analyzed by the quantum theory of atoms in molecules (QTAIM) and natural bond orbital (NBO) analysis. For QTAIM studies, wave functions which were generated by DFT method have been applied to be determined the quantum parameters such as electron density and its Laplacian at the bond critical points. Finally the calculated mean value of –U/G for this salt confirmed a non-covalent nature of O….H bonds.
KEYWORDS:EDB; Oxidation strength; Hydrogen bond; QTAIM; NBO
Download this article as:| Copy the following to cite this article: Khavani M, Izadyar M, Gholizadeh M, Housaindokht M. R. QTAIM and NBO Analysis of a New Oxidative Salt Of1,1/-(Ethane-1, 2-Diyl) Dipyridiniumbisiodate. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Khavani M, Izadyar M, Gholizadeh M, Housaindokht M. R. QTAIM and NBO Analysis of a New Oxidative Salt Of1,1/-(Ethane-1, 2-Diyl) Dipyridiniumbisiodate. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=4015 |
INTRODUCTION
Iodate is typically used in tropical climates, due to its good resistance to oxidation [1].The iodate anion has the capability to oxidize various functional groups.A diversity of salts with this anion, such as the potassiumsalt, have been prepared by Singh et al.[2] Potassium iodate is an oxidizing agent which can cause fires in the presence of the combustible materials or reducing agents.Potassium iodate is sometimes used for iodization of table salt to prevent the iodine deficiency, because iodide can be oxidized by molecular oxygen to iodine under wet conditions. Iodate salts are also available supported on the commercial anionic resinAmberlyst A26, and also on poly (vinyl pyridine) [3].
In this research we attempted to provide further insight into the quantum chemistry aspects of the electronic structure of this salt using the theoretical methods. A complementary study with the aim of elucidation the molecular properties associated with the oxidation strength is important in order to have a precise idea of the quantum reactivity indices.
Computational details
All calculations were performed with the Gaussian 09 software [4].As it has been reported that the DFT method by B3LYP function can reproduce good geometrical structures[5-8],the structures of the molecules were fully optimized and characterized as true minima by the absence of imaginary frequencies. Using the B3LYP/LANL2DZ level of the theory. Vibrational frequencies were determined to provide an estimation of the zero point vibrational energies (ZPVE).
Natural bond orbital analysis (NBO) analysis which is suggested by Reed et al.[9, 10]was carried out to explore the distribution of electrons intoatomic and molecular orbitals. Based on these data, donor–acceptor interactions ofEDB salt were fullyinvestigated.
For better analysis of electron density and bond orders evaluation of EDB, quantum theory of atom in molecule (QTAIM) was applied. QTAIM is a method that provides a rigorousand unambiguous criterion to determine which atoms are bonded, and which are separated, in a system[11].In this method, the topological properties of the charge density(ρ(r))is of interest and is summarized by its bond critical points (BCPs).These points refers to some places where the gradient vector field, 2ρ(r) vanishes. On the other hand, for determining the region in which the electroniccharge is locally depleted or concentrated, using the concept of the Laplacian ofthe electronic charge density ∇2ρ(r)is of importance. ∇2ρ(r)is positive,then there is a local depletion of charge; the electronic charge density is smallerthan the average density in the immediate neighborhood. But wherever∇2ρ(r) is negative, it means that there is a local concentration of charge. Since electron density is concentrated where∇2ρ(r)< 0 and it is depleted where∇2ρ(r) > 0the topology of the Laplacian is expressed in terms of L(r) = -∇2ρ(r)[12].
Results and Discussion
Structure Analysis
There is one independent crystallographically iodate anions for the salt of 1,1/-(ethane-1,2-diyl)dipyridiniumbisiodate (EDB) as shown in figures 1, in which I atoms are in a trigonal–pyramidal environment [13]. Two pyridine rings adopt an anti-conformation with respect to each other; the angle between these two rings is 3.84 degrees and C—H……O weak hydrogen bonds between the cations and anions lead to the formation of layers arranged parallel to the ab plane.
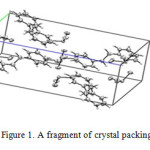 |
Figure1: A fragment of crystal packing of EDB |
According to crystallography structure [14], IO3– anions are on the top of the pyridine rings which is in spite of the theoretical data. Theoretical structure shows that IO3– anions are around the pyridine rings, according to figure 2.
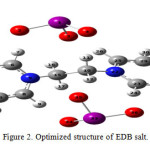 |
Figure2: Optimized structure of EDB salt: Click here to View Figure |
1,1/-(ethane-1, 2-diyl) dipyridiniumbisiodate salt structure was fully optimized at the B3LYP/LANL2DZ level of the theory. Table 1 shows the optimized geometrical parameters for this salt. According to table 1, OIO bond angles in therange of 100.90° to 105.4°. C—H….O hydrogen bonds between the cations and anions lead to the formation of layers which have been arranged parallel to the ab plane.
Table 1. Main geometric parameters in angstrom and degrees at the B3LYP/LANL2DZ level of EDB salt.
| Table 1. Main geometric parameters in angstrom and degrees at the B3LYP/LANL2DZ level of EDB salt. | |||
| parameter | Value | parameter | Value |
| I1 – O2 | 1.90 | O36 – I33 – O35 | 102.50 |
| I33 – O34 | 1.91 | O35 – I33 – O35 | 100.90 |
| C10 – N22 | 1.50 | O34 – I33 – O36 | 105.36 |
| C15 – N5 | 1.49 | N5 – C15 – C10 | 111.364 |
| C15 – H 17 | 1.09 | N22 – C10 – C15 | 109.18 |
| C15 – H16 | 1.09 | N5 – I33 – N22 | 48.92 |
| C10 – H 12 | 1.09 | N22 – C10 – C15 – N5 | 165.63 |
| O2 – I 1 – O3 | 102.73 | C10 – N22 – C23 – H24 | 3.11 |
| O2 – I 1 – O4 | 104.05 | N5 – C6 – O2 – I1 | -26.79 |
| O4 – I 1 –O3 | 100.94 | N22 – C23 – O36 – I33 | 13.22 |
Frequencies and Thermodynamic Analysis
Fundamental vibrational frequencies of EDB salt which are obtained from the theoretical calculationsat the B3LYP/LANL2DZ level, have been assigned and reported in table 2.
Table 2. Main vibrational frequencies (cm-1) of EDB salt at B3LYP/LANL2DZ.
| Table 2. Main vibrational frequencies (cm-1) of EDB salt at B3LYP/LANL2DZ. | |||
| Description | Frequencies | Description | Frequencies |
| I1 – O2 | 255.50 | O3 – O4 | 660.26 |
| I33 – O34 | 279.39 | C10 – C15 | 1044.81 |
| O35 – O36 | 635.60 | N22 – C10 | 1188.31 |
| O34 – O35 | 653.49 | C15 – N5 | 1206.43 |
Figures 3 and 4 provide the entropy and specific heat capacity values over a wide temperature range of 300 to 3000 K. Translational, rotational, and vibrational contributions to the molecular entropies and molecular specific heat capacity have been considered, and the results have been depicted in figures 3 and 4 respectively. Considering these figures, it has been confirmed that temperature dependent of vibrational entropy and specific heat capacity is more important than other contributions.
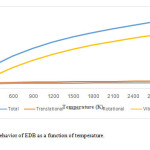 |
Figure3: Entropy behavior of EDB as a function of temperature.Click here to View Figure |
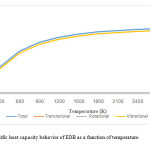 |
Figure4: Specific heat capacity behavior of EDB as a function of temperature. Click here to View Figure |
NBO Analysis
Charge distributions on EDB salt were calculated by the NBO method. Natural charges on EDB salt indicate that I atoms have positive character, while O and Natomshave negative ones. Negative characters for N and O atoms in the N-C-(H) ….O….I conjunction and positive character for H atom confirms the hydrogen bonding between two (three) molecules. Computed values of the natural charges for N, O and H have beenreported in table 3. According to table 3, positive character for H atom induces that to be attracted by O atom, because of the large value of negative charge density on the oxygen atom.
Table 3. Selected partial charges of EBD salt from the NBO analysis.
| Table 3. Selected partial charges of EBD salt from the NBO analysis. | |||
| Atom | Partial charge | Atom | Partial charge |
| I1 | 2.195 | N22 | -0.364 |
| O2 | -1.022 | H11 | 0.277 |
| O3 | -1.047 | H12 | 0.266 |
| O4 | -1.002 | H16 | 0.257 |
| N5 | -0.362 | H17 | 0.240 |
| C10 | -0.211 | I33 | 2.178 |
| C15 | -0.212 | O34 | -1.028 |
| O35 | -1.012 | O36 | -1.019 |
Delocalization of electron density between occupied Lewis-type (bonds or lone pairs) NBO orbitals and formallyunoccupied (anti-bonds and Rydberg’s) non-LewisNBO orbitals corresponds to a stabilizing donor–acceptorinteractions. The energy of these interactions has beencomputedby the second-order perturbation theory according toequation (1) [15].
E(2)= ∆Eij = qi {F2 (i, j) / (Ej – Ei ) (1)
Where qi is the donor orbital occupancy; Ej and Ei arediagonal elements and (i, j) are off-diagonal elementsassociated with NBO Fock matrix [16].Table 4 shows the energies of donor–acceptor interactionsfor EDB salt.
Table 4. Significant natural bond orbital interactions of the EDB salt.
| Table 4. Significant natural bond orbital interactions of the EDB salt. | |||||
| Donor | Acceptor | Delocalization Energy(kcal/mol) | Donor | Acceptor | Delocalization Energy(kcal/mol) |
| Lp O2 |
σ* I1 – O3 |
9.49 |
π C13 – C20 |
π* N5 – C8 |
44.21 |
| Lp O2 |
σ* I1 – O4 |
8.50 |
π N22 – C23 |
π* C25 – C29 |
21.08 |
| Lp O2 |
σ* C6 – H7 |
18.86 |
π C27 – C31 |
π* N22 – C23 |
13.80 |
| Lp O3 |
σ* C25 – H26 |
14.53 |
Lp O34 |
σ* C8 – H9 |
3.37 |
| Lp O4 |
σ* C10 – H11 |
5.40 |
Lp O35 |
σ* C10 – H12 |
4.30 |
| π N5 – C8 |
π* C6 – C18 |
21.07 |
Lp O36 |
σ* C23 – H24 |
11.24 |
| π C6 – C18 |
π* N5 – C8 |
13.60 |
Lp O34 |
σ* I3 – O35 |
14.36 |
According to table 4 the most important stabilizing effect is due to the strong orbitalinteractions of π C13 – C20 to π* N5 – C8 and π N22 – C23to π* C25 – C29. While the transmission between the LPO34 to σ*C8-H9 and LPO35 to σ*C10-H12 possesses the minimum stabilizing effects. Therefore during the oxidation process, it can be expected that the πtoπ* donor-acceptor interactions vary sharply.
QTAIM Analysis
QTAIM-based analysis of electron density at differentcharacteristic points, particularly at the bond critical points(BCP), is a powerful tool for investigation of differentChemical or physical phenomena[17-22]. It is well provedthat the magnitude of the electron density function estimatedat the BCP can reflect the strength of a given bond.The results of the topological analysis (Figure 5) for the electroniccharge density of the EDB salt, including thevalues of the electron density, ρ(r), and the Laplacian, L(r), have beenreported in table 5. Considering table 5, negative values of H-O bond laplacian such as H24-O36, H12-O36, H17-O34 represent an interaction between the nuclears andconcentration of electron density in these regions. Small values of laplacian are accordance to weak hydrogen bond at this critical bond points. Positive values of laplacian confirm the polar covalent bond nature of C-N and C-H bonds.
Table 5. Laplacian (∇2) and charge density (ρ) values of BCP for EDB salt.
| Table 5. Laplacian (∇2) and charge density (ρ) values of BCP for EDB salt. | |||||
| Bond | Charge density | Laplacian | Bond | Charge density | Laplacian |
| N22 – C23 |
0.282 |
0.121 |
C23 – H24 |
0.267 |
0.246 |
| N22 – C25 |
0.283 |
0.118 |
H26 – O3 |
0.038 |
-0.034 |
| H24 – O36 |
0.037 |
-0.034 |
N22 – C10 |
0.212 |
0.076 |
| H12 – O36 |
0.009 |
-0.008 |
C10 – C15 |
0.218 |
0.104 |
| H17 – O34 |
0.022 |
-0.021 |
N5 – C15 |
0.218 |
0.086 |
| H16 – O2 |
0.019 |
-0.018 |
N5 – C8 |
0.281 |
0.115 |
| H7 – O4 |
0.031 |
-0.021 |
N5 – C6 |
0.283 |
0.120 |
| H9 – O35 |
0.040 |
-0.036 |
C8 – H9 |
0.264 |
0.243 |
| C6 – H7 |
0.269 |
0.250 |
|||
In the theoretical charge density studies we have calculated the local potential energy density U(rBCP) and kinetic energy density G(rBCP) at BCPs essentially to represent a quantity proportional to the non-covalence bond energy. The average value of 0.93 for –G/U quantity, can reveal the hydrogen bond interactions, which previously confirmed by NBO analysis.
 |
Figure5: Molecular graph for the EDB salt using the QTAIM method. Click here to View Figure |
Conclusion
The molecular properties of the 1,1/-(Ethane-1,2-diyl)dipyridiniumbis(iodate) salt have been investigated using the B3LYP method with LANL2DZ basis set. Obtained resultsby using the quantum chemical calculations show the hydrogen bond formation in this salt. Vibrational frequencies have been investigated and the behavior of the entropies and the specific heat capacity of this salt in temperature range of 300 to 3000 K have been evaluated. For better understanding of the hydrogen bond importance during the salt formation NBO and QTAIM analysis were applied. Calculated interaction energies, E(2) by NBO method is accordance to (–U/G) values which have been obtained by AIM methods. Considering the NBO and QTAIM results, ti is expected that during the oxidation process, πto π* donor-acceptor interactions have the most important contribution.
Acknowledgement
Research Council of the Ferdowsi University of Mashhad is gratefully acknowledged for financial supports.
References
- Anonymous – Official Methods of Analysis, 14 th Edition Association of Official Analytical Chemists 33.147 Iodine in iodized salt, 1984.
- S. P. Singh, A. K. Singh, J. Mol. Catal. A Chem. 293, 97–102. 2008.
- B.Tamami, B. Parvanak-Borujeny,M. M. Khakzad, Iran. Polym. J.12, 331–338, 2003.
- M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery, T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez, J.A. Pople, Gaussian 03, Revision B.03, Gaussian. Inc., Wallingford, CT, 2009.
- P. Hohenberg,W. Kohn, Phys. Rev. B,136, 864, 1964.
- R. J.Bartlett, G. D. Purvis, Int. J. Quantum Chem. 14, 561, 1978.
- A.D. Becke, Phys. Rev. A 38 3098–3100, 1988.
- C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785–789, 1988.
- A.E. Reed, L.A. Curtiss, F. Weinhold, Chem. Rev. 88, 899, 1988.
- A.E. Reed, R.B. Weinstock, F. Weinhold, J. Chem. Phys. 78, 4066, 1983.
- R.F.W. Bader, Atoms in molecules, a quantum theory, Oxford University Press, New York, 1990.
- R.F.W. Bader, Chem. Rev. 91, 893, 1991.
- M. Gholizadeh, M. Pourayoubi, M. Kia, B. Notash, ActaCryst. E, 67 06714, 2011.
- Stoe& Cie. X-AREA, X-RED and X-SHAPE. Darmstadt, Germany, 2005.
- R.W.F. Bader, Chem. Eur. J. 12, 2896, 2006.
- M. Alajarin, M. Marin-Luna, M.Ortin, P. Sanchez-Andrada, A. Vidal, Tetrahedron, 67, 5590, 2011.
- Y. Aray, J. Rodriguez, D.J. Vega,J. Phys. Chem. B, 104, 4608, 2000.
- R.F.W. Bader, AIM2000 Program, ver 2.0, McMaster University, Hamilton, ON, Canada, 2000.
- C. Tratz, P.L. Fast, D.G. Truhlar, Phys. Chem. Comm. 2, 14, 1999
- Y. Zhao, D.G. Truhlar, Theor. Chem. Account 120, 215, 2008.
- E.A. Salter, G.W. Trucks, R.J. Bartlett, J. Chem. Phys. 90, 1752, 1989.
- C.F. Matta, R.W.F. Bader, J. Phys. Chem. A 110, 6365, 2006.

This work is licensed under a Creative Commons Attribution 4.0 International License.









