One-Pot Synthesis of 1,8-Dioxo-Octahydroxanthene Derivatives Using Zn(No3)2 Under Solvent-Free Conditions
Seyed Amir Hasan Vahabi *, Farhad Hatamjafari, Khalil Pourshamsian
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran
DOI : http://dx.doi.org/10.13005/ojc/300262
Article Received on :
Article Accepted on :
Article Published : 12 Apr 2014
Zn(NO3)2 as an efficient catalyst have been used for the one-pot synthesis of 1,8-Dioxo-octahydroxanthene Derivatives via multi-component reactions between dimedone and various aromatic aldehydes under solvent-free conditions. The presented method is available, environmentally friendly, cheap and highly effective to give the products in good to excellent yields.
KEYWORDS:Xanthenes; Solvent-free; Multicomponent reactions; Zn(NO3)2.
Download this article as:| Copy the following to cite this article: Vahabi H. A. S, Hatamjafari F, Pourshamsian K . One-Pot Synthesis of 1,8-Dioxo-Octahydroxanthene Derivatives Using Zn(No3)2 Under Solvent-Free Conditions. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Vahabi H. A. S, Hatamjafari F, Pourshamsian K. One-Pot Synthesis of 1,8-Dioxo-Octahydroxanthene Derivatives Using Zn(No3)2 Under Solvent-Free Conditions. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=2939 |
INTRODUCTION
Multicomponent reactions (MCRs) refers to a reaction in which two or more ingredients are combined within a single process and the products they create, which is part of all the components are present1-3. Since the multi-component reactions for the synthesis of organic compounds and these compounds can be used as a drug and precursor multicomponent reactions, so to investigate them out is important4.
In our ongoing research prompted by our interest in multiple component reactions and as part of programs in the area of heterocyclic compounds containing oxygen, and due to the resultant pharmacological interest in compounds which belong to the xanthene Derivatives, although this reaction done5.
Xanthene derivatives are one of the important classes of organic compounds which are biologically important drug intermediates in the field of medicinal chemistry for their biologically active properties, such as antimalarial, antibacterial, antiinflammatory, and antiviral properties and have been used as dyes, fluorescent material and in laser technologies6-9. Recently, several improved methodologies have been developed that use triethylbenzyl ammonium chloride10, p-dodecyl benzenesulfonic acid11, phosphomolybdic acid supported on silica gel12, sulfonic acid on silica gel13, HClO4–SiO2 14 and ZnO 15 among others. Previously, we have synthesized a number of heterocyclic compounds16-26.
In this study, we have used of Zn(NO3)2 as a catalysts to develop a new and easy methodology for the synthesis of xanthene derivatives. The experiments were started with the study of one pot reaction, a short time with high yields, easy separation of product, and a 3-component method, mild and efficient method for the preparation of the xanthenes by using Zn(NO3)2 as a catalyst (Scheme1).
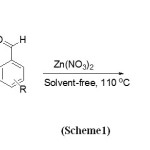 |
Scheme 1 Click here to View Scheme |
EXPERIMENTAL
All chemicals were obtained from Merck or Fluka without further purification. Silica gel SILG/UV 254 plates were used for TLC. IR spectra were measured on a Shimadzu IR-470 Spectrophotometer. 1H NMR spectra were determined on Bruker 400 DRX AVANCE instrument at 400 MHz, respectively.
General procedure for preparation of A1
A mixture of aldehyde (1 mmol), dimedone (2 mmol), and Zn(NO3)2 (6 mol %) as a catalyst was stirred at 110 oC for 10 min. The progress of reaction was monitored by TLC. After finishing, recrystallized from ethanol 95% to give pure products (A1)
Spectral data
3,4,6,7-tetrahydro-3,3,6,6-tetramethyl-9-(3-nitrophenyl)-2H-xanthene-1,8(5H,9H)-dione (A1)
White crystals, Yield: (90%), mp 171-174oC. IR (νmax/cm-1) (KBr): 3074 (arom. CH Str.); 2990 (aliph. CH Str.); 1660 (C=O Str.); 1622 (C=C Str.); 1500, 1360 (NO2,Str). 1H NMR (400.22 MHz CDCl3): δ=1.00 (6H, s, 2CH3); 1.12 (6H, s, 2CH3); 2.19 (4H, ABq, 3J=16.4Hz, 2CH2); 2.52 (4H, s, 2CH2); 4.84 (H, s, CH); 7.28 8.04- (4H, m, CH).
RESULTS AND DISCUSSION
We have been able to introduce an efficient and environmentally friendly for the synthesis of xanthene derivatives via condensation of dimedone with various aromatic aldehydes and ammonium acetate. Therefore, reported Zn(NO3)2 as catalyst which could provide an efficient, cheap, easy separation, high yield and simple route under solvent-free condition for the synthesis of 1,8-Dioxo-octahydroxanthenes.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
REFERENCES
- Domling A., Chem. Rev., 106: 17 (2006).
- Khan A. J and Basheer M., Orient. J.Chem., 27(4): 1759-1762 (2011).
- Setamdideh D., Karimi Z and Rahimi F., Orient. J.Chem., 27(4): 1621-1634 (2011).
- Kalinski C., Lemoine H., Schmidt J., Burdack C., Kolb J., Umkehrer M. and Ross G., Syn. lett., 24: 4007 (2008).
- Peet N. P., Huber, E.W. and Huffman, J. C., J. Heterocycl. Chem., 32: 33 (1995).
- Schumacher K., Ravikovitch P.I., Du Chesne A., Neimark A. and Unger K. K., Langmuir 16: 4648(2000)
- Chibale K., Visser M., Schalkwyk D. V., Smith P. J., Saravanamuthu A. and Fairlamb A. H., Tetrahedron 59(13): 2289 (2003)
- Hideo T., Jpn. Tokkyo Koho JP 56005480., Chem. Abst., 95: 80922b (1981).
- Poupelin J. P., Saint-Rut G., Fussard-Blanpin O., Narcisse G., Uchida-Ernouf G. and Lakroix R., Eur. J. Med. Chem., 13: 67 (1978).
- Wang X. S., Shi D. Q., Li Y. L., Chen H., Wei X. Y. and Zong Z. M., Synth Commun., 35, 97 (2005).
- Jin T. S., Zhang J. S., Xiao J. C., Wang A. Q. and Li T. S., Synlett., 866 (2004) .
- Srihari P., Mandal S. S., Reddy J. S. S., Srinivasa Rao R. and Yadav J. S., Chin Chem Lett., 19, 771 (2008).
- Mahdavi G. H., Bigdeli M. A. and Saeidi Hayeniaz Y., Chin Chem Lett., 20, 539 (2009).
- Kantevari S., Bantu R. and Nagarapu L., J Mol Catal A: Chem., 269, 53 (2007).
- Maghsoodlou M. T., Habibi-Khorassani S. M., Shahkarami Z., Maleki N. and Rostamizadeh M., Chin Chem Lett., 21, 686 (2010).
- Azizian J., Hatamjafari F., Karimi A. R. and Shaabanzadeh M., Synthesis. 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F. and Mohammadizadeh M.R., Arkivoc. (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications. 36: 3563 (2006).
- Azizian J., Hatamjafari F. and Karimi A. R., Journal of Heterocyclic Chemistry. 43: 1349 (2006).
- Hatamjafari F and Montazeri N., Turkish Journal of Chemistry. 33: 797 (2009).
- Hatamjafari F., Orient. J. Chem., 28: 141(2012).
- Hatamjafari F., Orient. J. Chem., 29: 93(2013).
- Hatamjafari F and Alijanichakoli F., Orient. J.Chem., 29: 145(2013).
- Hatamjafari F and Hosseinian A., Orient. J.Chem., 29: 109(2013).
- Hatamjafari F and Khojastehkouhi H., Orient. J. Chem., 30: (2014 in press).
- Hatamjafari F and Germani Nezhad F., Orient. J. Chem., 30: (2014 in press).

This work is licensed under a Creative Commons Attribution 4.0 International License.









