Nano Tio2: An Efficient Catalyst for The synthesis of Biscoumarins in Aqueous Medium
Hamide Babaei* and Naser Montazeri
Department ofchemistry, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran
DOI : http://dx.doi.org/10.13005/ojc/300223
Article Received on :
Article Accepted on :
Article Published : 16 Apr 2014
An easy method for the synthesis of biscoumarins through a one-pot reaction of 4-hydroxycoumarin and aryl aldehydes under very mild reaction conditions using nano TiO2 as catalyst in aqueous medium is described. The products were obtained in short reaction times with excellent yields under reflux. This method is of great value because of its environmentally benign character, high yield processing, and easy handling. We believe this applicability of nano-TiO2 with mentioned advantages makes our method superior over all previous reported methods to the synthesis of biscoumarins.
KEYWORDS:Biscoumarins; aqueous medium; 4-hydroxycoumarin; nano-TiO2; aryl aldehydes
Download this article as:| Copy the following to cite this article: Babaei H, Montazeri N. Nano Tio2: An Efficient Catalyst for the synthesis of Biscoumarins in Aqueous Medium. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Babaei H, Montazeri N. Nano Tio2: An Efficient Catalyst for the synthesis of Biscoumarins in Aqueous Medium. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3078 |
INTRODUCTION
Coumarin derivatives and especially biscoumarins have long been the subject of numerous studies on account of their pharmacological and biological properties1-7. Compounds with these ring system have diverse pharmaceutical activities such as antioxidant, antimicrobial, anticoagulant, anti-HIV, antithrombotic, antieancer, enzyme inhibitory, urease inhibitory and cytotoxicity8-16. A number of biscoumarins are useful as malignant melanoma, metastatic renal cell carcinoma and prostate cancer drugs17-19.In view of different biological and chemical applications of biscoumarins, the development of suitable synthetic methodologies for their generation has been a topic of great interest in recent times. The general method for synthesis of biscoumarin derivatives involves the reaction of 4-hydroxycoumarin with aryl aldehydes in the presence of different catalysts such as Zn(proline)220, nano SiO2Cl21, K2CO3 in acetic anhydride22, ruthenium(III) chloride hydrate23, Et2AlCl in acetonitrile or dichloromethane at room temperature24, piperidine9, molecular iodine25, sodium dodecyl sulfate(SDS)26, phosphotungstic acid27, heteropolyacids28, tetrabutylammonium bromide(TBAB)29, 1,8-diazabicyclo[5.4.0] undec-7-ene(DBU)30, POCl3 in dry DMF31, andmanganous chloride32. However, some of these procedures have disadvantages, for example tedious work up and use of expensive reagents in organic solvents. These problems prompted us towards further investigation in search for a new catalyst, which will carry out the synthesis of biscoumarins under simpler experimental set up and environmentally friendly conditions. In this article, we present a one-pot reaction for the preparation of biscoumarins derivatives in the presence of nano TiO2 in aqueous medium(Scheme1).
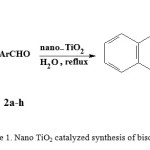 |
Scheme 1. Nano TiO2 catalyzed synthesis of biscoumarins |
EXPERIMENTAL
All of the chemical material used in this work purchased from Fluka or Merk and without further purification. Melting points were recorded on an Electro thermal type 9100 melting point apparatus. The IR spectra were obtained on a 4300 Shimadzu spectro photometer in KBr disks. The1H NMR(500 MHz) spectra were recorded on a Bruker-Ac-500 spectrometer.
General procedur for the synthesis of biscoumarins (3a-h):
A solution of 4-hydroxycoumarin 1 (2mmol), an aromatic aldehyde 2a-h (1 mmol) and nano TiO2(5 mol% based on aromatic aldehyde) in H2O (10 ml) was heated on the oil bath under reflux for the time period as indicated in table 2. Theprogress of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature and the solid product was collected by filtration and washed with cold water. The solid residue was diluted with chloroform (5 ml) and the catalyst was separated. The filtrate was evaporated on rota-evaporator to give a solid which was dried and recrystallized from ethanol to afford pure products 3a-h in high yields. All the products were identified by comparing the analytical data(Melting point, IR, H NMR) with those reported or with authentic samples prepared by the conventional method, in which we used nano TiO2 as the catalyst. The results are summarized in the table 2.
RESULT AND DISCUSSION
To initiation our study the reaction of4-hydroxycoumarin with benzaldehyde were employed as a model reaction to examine the effect of various solvents such as EtOH, MeOH, CHCl3 and H2O and varying amount of nano TiO2 (5, 10, 15 and 20mol%) as catalyst. In an optimized reaction conditions, 4-hydroxycoumarin (2 mmol) and benzaldehyde (1 mmol) in H2O (10 ml) were mixed in the presence of nano TiO2 (0.05 mmol) for 5 min. The reaction proceeds very cleanly under reflux and was free of side products. After completion of the reaction (monitored by TLC), a simple work up affords the products in high yields (Table 2). Among the solvents tested, the reaction in chloroform using 5 mol% of the catalyst gave a low yield of the desired product. Ethanol and methanol gave moderate to good yields under this conditions. However, the reaction in H2O with 5mol% of catalyst afforded product 3a in 96% yields. Using more than 5 mol% of catalyst, has less effect of the yield and time of the reaction.
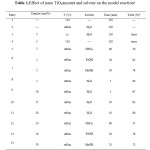 |
Table 1.Effect of nano TiO2amount and solvent on the model reactiona Click here to View table |
As shown in table 1, no desirable products could be detected in the absence of catalyst for 120 min, which indicated that the catalyst should be absolutely necessary for this reaction. Only a trace product was obtained in the solvent-free conditions in the presence of 5 mol% of the catalyst even at 110°C. After optimization of the reaction conditions to experimental trials illustrating this method for the synthesis of derivatives were conducted.The results are summarized in table 2.
 |
Table 2.Nano TiO2 catalyzed synthesis of biscoumarins 3a-ha Click here to View table |
a2mmol 4-hydroxycoumarin, 1 mmol aryl aldehyde and 5 mol% nano TiO2 in H2O (10 ml) under reflux. bThe products were characterized by comparison of their spectroscopic and physical data with authentic samples synthesized by reported procedures. cIsolated yield.
Benzaldehyde and other aromatic aldehydes containing electron-withdrawing groups (such as halide and nitro groups) or electron-donating groups (such as hydroxyl, methyl and methoxy groups) were employed which were found to react well to give the corresponding biscoumarin derivatives in high yields.
CONCLUSIONS
In conclusion, we have described a highly efficient one-pot synthesis for the preparation of biscoumarin derivatives in the reaction of 4-hydroxycoumarin and aryl aldehydes in the presence of nano TiO2 as catalyst. Easy work up, short reaction time, environmentally, ready commercial availability of the catalyst and high yields, make the procedure an attractive alternative to the existing methods for the synthesis of biscoumarin derivatives.
ACKNOWLEDGMENTS
We gratefully acknowledgements the financial support of this research by Islamic Azad University, Tonekabon Branch.
REFERENCES
- S. Stanchev, G. Momekov, F. Jensen, I. Manolov, Eur. J. Med. Chem., 20:1(2007).
- J. C. Jung, J. H. Lee, S. Oh, J. G. LLeed, O. S. Parkeb, Bioorg. Med. Chem. Lett.,14:5527(2004).
- N. Hamdi, M. C. Puerta, P. Valerga, Eur. J. Med. Chem.,43:2541(2008).
- J. Yamashita, S. Takeda, H. Matsumoto, N. Unemi, M. Yasumoto, Chem. Pharm. Bull.,35:2373(1987).
- V. D. Kancheva, V. P. Boranova, J. J. Nechev, I. I. Manolov, Biochimie.,92:1138(2010).
- I. Manolov, C. Maichle-Moessmer, N. Danchev, Eur. J. Med. Chem.,41:882(2006).
- J. C. Jung, O. S. Park, Molecules.,14:4790(2009).
- I. Kostova, G. Momekov, M. Zaharieva, M. Karaivanova, Eur. J. Med. Chem.,40:542(2005).
- K. M. Khan, S. Iqbal, M. A. Lodhi, G. M. Maharvi, Zia-Ullah, M. I. Choudhary, Atta-ur-Rahman, S. Perveen, Bioorg. Med. Chem.,12:1963(2004).
- M. I. Choudhary, N. Fatima, K. M. Khan, S. Jalil, S. Iqbal, Atta-ur-Rahman, Bioorg. Med. Chem.,14:8066(2006).
- P. C. M. Mao, J. F. Mouscadet, L. Leh, C. Auclair, L. Y. Hsu, Chem. Pharm. Bull.,50:1634(2002).
- H. Zhao, N. Neamati, H. Hong, A. Mazumder, S. Wang, S. Sunder, G. W. A. Milne, Y. Pomier, T. R. Burke, J. Med. Chem.,40:242(1997).
- Z. H. Chohan, A. U. Shaikh, A. Rauf, C. T. Supuran, J. Enzyme Inhib. Med. Chem.,21:741(2006).
- A. Maucher, E. J. Von Angerer, J. Cancer Res. Clin. Oncol.,120:502(1994).
- M. E. Marshall, J. L. Mohler, K. Edmonds, B. Williams, K. Bulter, M. Ryles, L. Weiss, D. Urban, A. Beuschen, M. Markiewicz, G. Cloud, J. Cancer Res. Clin. Oncol.,120:S39(1994).
- B. Musicki, A. M. Periers, P. Laurin, D. Ferroud, Y. Benedetti, S. Lachaud, F. Chatreaux, J. L. Haesslein, A. Itlis, C. Pierre, Bioorg. Med. Chem. Lett.,10:1695(2000).
- M. E. Marshall, K. Bulter, A. Fried, Mol. Biother., 3:170(1991).
- R. D. Thornes, L. Daly, G. Lyneh, B. Breslin, H. Browne, H. Y. Browne, T. Corrigan, P. Daly, G. Edwards, E. Gaffney, J. Cancer Res. Clin. Oncol.,120:S32(1994).
- J. L. Mohler, L. G. Gomella, E. D. Crawford, L. M. Glode, C. D. Zippe, W. R. Fair, M. E. Marshall, Prostate., 20:123(1992).
- Z. N. Siddiqui, F. Farooq, Cat. Sci. & Tech.,1:810(2011).
- R. Karimian, F. Piri, A. A. Safari, S. J. Davarpanah, J. Nano. Chem.,3:52(2013).
- R. Gasparova, K. Kotlebova, M. Lacova, Nava Biotech.,9-3:349(2009).
- K. Tabatabaeian, H. Heidari, A. Khorshidi, M. Mamaghani, N. O. Mahmoodi, J. Serb. Chem. Soc.,7(4):407(2012).
- H. Hagiwara, S. miya, T. Suzuki, M. Ando, I. Yamamoto, M. Kato, Heterocycles.,51:493(1999).
- M. Kidwai, V. Bansal, P. Mothsra, S. Saxena, R. K. Somvanshi, S. Dey, T. P. Singh, J. Mol. Catal., A 268:76(2007).
- H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc.,7:890(2010).
- P. Singh, P. Kumar, A. Katyal, R. Karla, S. K. Dass, S. Prakash, R. Chandra, Catal. Lett.,134:303(2010).
- M. M. Heravi, S. Sadjadi, N. Mokhtari Haj, H. Oskooie, F. F. Bamoharram, Catal. Commun.,10:1643(2009).
- J. M. Khurana, S. Kumar, Tetrahedron Lett.,50:4125(2009).
- H. Hagiwara, N. Fujimoto, T. Suzuki, M. Ando, Heterocycles.,53:549(2000).
- M. H. A. Elgamal, N. M. M. Shalaby, M. A. Shaban, H. Duddeck, B. Mikhova, A. Simon, G. Toth, Monatsh. Chem.,128:701(1997).
- J. N. Sangshetti, N. D. Kokare, D. B. Shinde, Green Chem. Lett. Rev.,2:233(2009).

This work is licensed under a Creative Commons Attribution 4.0 International License.









