Functionalization of carboxylated multiwall nanotubes with dapsone derivatives and study of their antibacterial activities against E.coli and S. aureus
Javad Azizian1*, Malak Hekmati1, Orkideh Ghorban Dadras2
1Department of Chemistry, Science and Research branch, Islamic Azad University, Tehran, Iran 2Department of Medicinal Chemistry, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/300236
Article Received on :
Article Accepted on :
Article Published : 27 Jun 2014
Bacteria are becoming resistant to the antibiotics prescribed and therefore new treatments are essential to be developed. Multiwall carbon nanotubes (MWNTs) have interesting antibacterial activities and offering a promising new treatment preventing bacteria from becoming resistant. With this aim, we report for the first time three novel modified carboxylated multiwall carbon nanotubes consisting of MWNT-Dapsone, MWNT-Dapsone-imine and MWNT-Dapsone-imine. Copper complex. The functionalized carboxylated multiwall nanotubes were then characterized by FT-IR, Raman, TEM. Moreover, the antibacterial activity of the MWNT-COOH (A), MWNT-dapsone (B), MWNT-Dapsone-imine (C) MWNT-Dapsoneimine.Copper complex (D) , Dapsone (E(, Dapsone-imine (F), has been investigated against gram-negative Escherichia coli and gram-positive Staphylococcus aureus. These results show that compund F exhibited significant antibacterial activity and have a potential to be used as antibacterial agent.
KEYWORDS:Carboxylated multiwall nanotubes; Dapsone; Functionalization, Characterization; Antibacterial activity
Download this article as:| Copy the following to cite this article: Azizian J, Hekmati M, Dadras O. G. Functionalization of carboxylated multiwall nanotubes with dapsone derivatives and study of their antibacterial activities against E.coli and S. aureus. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Azizian J, Hekmati M, Dadras O. G. Functionalization of carboxylated multiwall nanotubes with dapsone derivatives and study of their antibacterial activities against E.coli and S. aureus. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3966 |
Introduction
Antimicrobial resistance is fast becoming a major problem with rapid increases in multidrug-resistant bacteria (Saha et al., 2009). In general, bacteria have the genetic ability to send and acquire resistance to drugs, which are used as pharmacologic agents (Faúndez et al., 2004). The microbial resistance represent a global concern and the outlook for the use of antimicrobial drugs in the future is still uncertain (Stanila et al., 2011; Chaudhary et al.,2010).
Recently, carbon nanotubes (CNTs) have attracted increasing attention in biomedical fields due to their unique structure and properties, including high aspect ratios, large surface areas, nanosized stability and rich surface chemical functionalities (Wu et al., 2011; Azizian et al., 2013). With these unique structures and properties, CNTs have been developed as promising nanoplatforms to immobilize biological or therapeutic molecules, such as proteins, antibodies, siRNA or drugs on their surface, and especially these functionalized CNTs are capable of crossing biological barriers independently of the cell type, which makes them suitable candidates for drug delivery systems.(Tian et al., 2011; Hu et al., 2009; Holzinger., 2001). The chemical modification of carbon nanotubes has received significant attention in recent decades (Chen et al., 1998; Hamon et al., 1999). Chemical functionalization is a common technique to increase dispersion stability and biocompatibility of CNTs (Georgakilas et al., 2002; Sun et al., 2002). CNT have been proposed as multipurpose innovative transporters for drug delivery since they can be covalently or non-covalently attached to drug molecules and carry them throughout the body in a biocompatible way (Karchemski et al., 2012; Entezari et al., 2013). Modified carbon nanotubes have been widely studied for their antibacterial, antifungal and potential cytotoxic chemotherapeutic agents. Many factors may influence the antibacterial activity of carbon nano materials, including: electronic structure, size and surface chemical properties, as well as the interacting conditions between carbon nanomaterials and bacterial cells.(Liu et al., 2012). More recently, transition metal complexes have attracted attentions of inorganic, metallo-organic as well as bio-inorganic chemists because of their structural diversity, antibacterial activity and enormous number of biological applications (Safari et al., 2013; Yousefi et al., 2012). The ability of metal to combine with ligands and then release ligand in specific process make them ideal candidate for use in biological system. It is well known that synthetic copper(II) complexes have been reported to act as potential anticancer and antibacterial agents (Tella and Obaleye, 2009 ). The pharmacological activities of these metal complexes depend on the metal ion, organic ligands and the structure of the compounds (Yousefi et al., 2014). Dapsone ((4, 4’-diaminodiphenylsulphone), a sulphone analog, is the main antileprosy drug because of its inherent level of bactericidal activity, easy application and minimal side effects (Makarov et al ., 2006; Wadher et al., 2009). As an extension of this research field, we aimed to attach the new pharmacologic agents to the surface of carboxylated multiwall nanotubes, in order to take advantage of the MWNT’s outstanding biological properties and to screen the final products for their antibacterial activity.
Result and discussion
Infrared spectroscopy
FT-IR spectroscopy was used to identify the chemical groups that were attached to MWNTs. Figure 1 shows the FT-IR spectra of MWNT-COOH (A), MWNT-dapsone (B), MWNT-Dapsone-imine (C) MWNT-Dapsoneimine.Copper complex (D).
In spectrum (a), the band at around 1568 cm-1 corresponds to the stretching mode of the C=C double bond that forms the framework of the carbon nanotube sidewall . The peaks at 1705 and 2450 cm-1 to 3400 cm-1 apparently corresponds to the stretching modes of the carboxylic acid groups. The two bands at around 2850 cm-1 which are seen in all spectrums are attributed to the CH stretching of MWNT–COOH defects. In spectrum (b), the three bands at 3143 cm-1, 3304 cm-1 and 3381 cm-1 can be assigned to the N-H stretching modes and carbonyl peak in the spectrum (b) shift to 1656cm-1 (as compared with 1705 cm-1 in spectrum (a)) is a result of amide (C =O)NH linkage formation. This could indicate that amidation reactions occurred between the amino groups of dapsone and the carboxyl groups on the surfaces of the MWNTs. In the spectrum (c) the new strong peak at 3429 cm-1 can be assigned to the O-H stretching modes. The carbonyl peak in the spectrum (c) shift to 1660 cm-1 (as compared with 1705 cm-1 in spectrum (a)) is a result of amide (C =O)NH linkage formation and so the formation of a new peak in 1625 cm-1 indicating the presence of C=N double bond This is certainly an indication for the formation of 2-((4-(4-aminophenylsulfonyl)phenylimino)methyl)phenol on the surfaces of the MWNTs. In spectrum (d), the broad band at around 1588 cm-1 corresponds to the stretching mode of the (C =O). The peak at 1625 cm-1 presents in compound (C) was shifted to lower frequency of 1463 cm-1 in compound (D), respectively, thus indicating coordination of the metal ion to N atom. Furthermore, the nature of the metal-ligand bonding is confirmed by the newly formed bands at ~ 543 cm-1 in the spectra of this compound, which is tentatively assigned to Cu-O vibration.
 |
Figure1: FT-IR spectra of MWNT–COOH (A) and functionalized MWNTs (B-D) Click here to View Figure |
Raman Spectroscopy
Raman spectra offer useful information concerning the slightly structural changes of MWNTs, especially the changes owing to significant sidewall modification. As can be seen in Figure 2, the characteristic peaks of MWNT tangential modes, namely the D band at around 1320 cm−1 and the G band at around 1590 cm−1 slightly changed. We observed an increase in the ratio of intensities R = ID/IG in modified nanotubes. This indicates an increased disorder of the graphitic structure of the modified nanotubes, which shows that the nanotubes were covalently modified ( Dresselhaus et al., 2005).
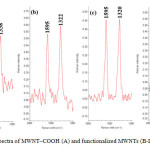 |
Figure2: Raman spectra of MWNT–COOH (A) and functionalized MWNTs (B-D) . Click here to View Figure |
TEM Characterization of the modified MWNTs
Figure 3 presents TEM images of the MWNT–COOH (A) and the modified MWNTs (B-D). The MWNT–COOH show almost smooth surface (a) while increased roughness of the functionalized CNT surfaces evident in TEM images of MWNT-Compounds.
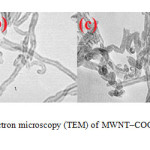 |
Figure3: Transmission electron microscopy (TEM) of MWNT–COOH (A) and functionalized MWNTs (B-D).Click here to View Figure |
Biological studies
Antibacterial activity
The antibacterial activity of MWNT-COOH (A) as well as corresponding functionalized MWNTs (B-D) and compound E and F was performed against one Gram positive (Staphylococcus aureus) and another Gram negative (Escherichia coli) bacteria, and the results are summarized in Table 1. The results indicates that the modified MWNTs show higher activity than MWNT-COOH against these two bacteria (Fig 4). These data also demonstrate that MWNT-COOH has no activity on the growth of S. aureus bacteria. Compound E possessed a significant inhibitory effect against all the tested bacteria. The increase of the zone of inhibition for compound C when compared with corresponding ligands (compound F) is an indication that the modified MWNTs is able to decrease the population of Staphylococcus aureus bacteria. Moreover, Compounds (D) present higher activity than other functionalized MWNTs against E. coli. Therefore, it should be noted that presence of metal ion in the structure of compound D had a great influence on the suppression activity of this compound on E.Coli. Noticeably, The antibacterial activity of the compounds F and C against E.Coli were in a similar range. An improved activity was observed for the functionalized MWNTs of the present investigation. As these results are preliminary, further study on the antibacterial activity of these complexes is highly recommended.
Table 1. Antibacterial screening data of Compound A-E against the tested bacteria (inhibition zone in mm)
|
Compound |
Concentration |
E. coli |
S. aureus |
|
Compound (A) |
1000 ppm |
8 |
0 |
|
Compound (B) |
1000 ppm |
14 |
10 |
|
Compound (C) |
1000 ppm |
12 |
15 |
|
Compound (D) |
1000 ppm |
16 |
8 |
|
Compound (E) |
1000 ppm |
42 |
22 |
|
Compound (F) |
1000 ppm |
12 |
10 |
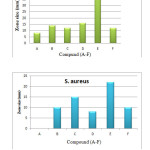 |
Figure4: Average diameter of zones of inhibition for compound (A-F) against E.Coli and S. aureus bacteria. |
Experimental
Material and methods
All chemicals and reagents were purchased from Merck and used without further purification. MWNT– COOH (95 % purity, 20–30 nm; Netrino Co. Ltd) were purchased and used as received. The FT-IR spectra were recorded on a Nexus 870 FT-IR spectrometer using KBr pellets (Thermo Nicolet, Madison, WI). FT-Raman spectra were recorded on 960 ES spectrometer (Thermo Nicolet). TEM measurement was carried out on the LEO 912 AB electron microscope.
Preparation of MWNT-COCl
60 mg of the MWNT-COOH were sonicated in 90 ml of DMF for 40 min to give a suspension. Oxaly- chloride (2.5 ml) was added drop-wise to the MWNT suspension at 0 ˚C under N2. The mixture was stirred at 0 ˚C for 2 h and then at room temperature for another 2 h. Finally, the temperature was raised to 70˚C and the mixture was stirred overnight to remove excess oxalyl chloride (Figure 5). After cooling to room temperature, the mixture was filtered through 0.2 µm pore size polytetrafluoroethylene membrane. The filtrate was washed with EtOH (3*100 ml), and then dried in an oven for 4 h at 60 °C.
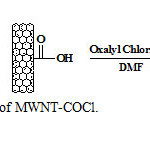 |
Figure5: Preparation of MWNT-COCl. Click here to View Figure |
Preparation of MWNT–CO-4-(4-aminophenylsulfonyl)benzenamine (MWNT-D)
250 mg of dapsone compound was sonicated in 90 ml of DMF for 30 min and added to suspension 50 mg MWNT-COCl, and the mixture was refluxed at 100 ºC for 48 h (Figure 6). Then the mixture was cooling to room temperature and filtered through 0.2 µm pore size polytetrafluoroethylene membrane. The filtrate was washed with MeOH (3*100 ml) and THF (100 ml). Subsequently, the black solid was vacuum dried at room temperature.
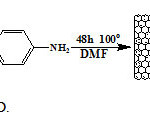 |
Figure6: Preparation of MWNT-D. Click here to View Figure |
Preparation of MWNT–CO-2-((4-(4-aminophenylsulfonyl)phenylimino)methyl)phenol (MWNT-DS)
250 mg of dapsone compound sonicated in 90 ml of DMF for 30 min and was added to suspension 50 mg MWNT-COCl, and the mixture was refluxed at 100 ºC for 48 h. Then the mixture was cooling to room temperature. To the mixture cooled was added 300 mg salicylaldehyde and then the mixture was stirred at room temperature for another 24 h (Figure 7). After completion of the reaction, the mixture was filtered and washed thoroughly with MeOH (3*100 ml) and THF (100 ml). Subsequently, the black solid was vacuum dried at room temperature.
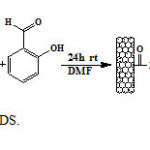 |
Figure7: Preparation of MWNT-DS. Click here to View Figure |
Preparation of MWNT–CO-[2-((4-(4-aminophenylsulfonyl)phenylimino)methyl)phenol][CuCl2] (MWNT-DSC) 0.2 g of nano.dapsone.imin were sonicated in 30 ml of N,N-dimethylformamide (DMF) for 30 min. methanolic solution of CuCl2. 2H2O (0.1g) was added dropwise to the suspension Nano.dapsone.imin (Fig 8). The mixture was sonicated for one hour and stirred at room temperature for 24 h. Finally, the mixture was filtered and washed thoroughly with tetrahydrofuran. Subsequently, the black solid was vacuum-dried at room temperature for 5 h.
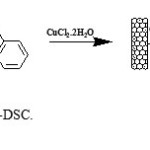 |
Figure 8. Preparation of MWNT-DSC. Click here to View Figure |
Biological studies
All of the synthesized compounds were screened for their antibacterial activity against two strains of bacteria ( Escherichia coli and Staphylococcus aureus ). The antibacterial activities of the ligand and metal complexes were carried out using well diffusion method. Muller Hilton Agar was prepared and sterilized. 20 ml of media was poured into the petri-dishes and allowed to solidify. The wells were made carefully and these were completely filled with the test solutions. The test solutions of the studied compounds were prepared in DMF at a concentration of 1,000 μg/mL. The plates were incubated immediately at 37◦C for 24 hours and the diameter of the inhibiting area around each well was estimated, which is described as the inhibiting effect against bacteria. The average of three diameters was calculated for each sample.
Conclusions and outlook
In summary, we have introduced pharmacologic agents onto the surface of MWNTs in order to observe the effect of different substituents attached to the surface of MWNTs on the studied bacteria. The functionalized MWNTs have been characterized by FT-IR and Raman spectroscopies and TEM. The antibacterial activity of the synthesized compounds has been evaluated against E.coli and S. aureus. Functionalized MWNTs showed the ability to inhibit E.coli and S. aureus. The most outstanding results were obtained from the activity of Compound F against E.coli, which exhibited much higher antibacterial activity than other modified MWNTs. Therefore, compund F seems to be a very promising candidate for further tests which will be carried out in the near future.
Acknowledgments
The authors like to express their profound gratitude to the Research Council of Islamic Azad University Science and Research branch, Iran for providing necessary research facilities and financial assistance.
References
- Azizian J, Ardestani E, Entezari M (2013) Modification and Functionalization of Carboxylated Multiwall Nanotubes with Amantadine, Pregabalin and Alendronate Using Amidation as Anti Cancer Derivatives. Curr. Nanosci.9: 442-446.
- Chen J, Hamon MA, Hu H, Chen Y, Rao AM, Eklund PC, Haddon RC (1998) Solution Properties of Single-Walled Carbon. Nanotubes Sci 282: 95-98.
- Chaudhary M, Pareek D, Ojha KG, Pareek A (2010) Synthesis and antibacterial activity of 1-(2-Diazo- 6-ethoxybenzothiazolyl) substituted benzene derivatives. International Journal of Current Chemistry 1: 175-179.
- Dresselhaus MS, Dresselhaus G, Saito R, Jorio A (2005) Raman spectroscopy of carbon nanotubes. Physics Reports-Review Section of Physics Letters 409: 47-99.
- Entezari M , Safari M , Hekmati M, Hekmat S, Azin A (2013) Modification of carboxylated multiwall nanotubes with benzotriazole derivatives and study of their anticancer activities. Med Chem Res. DOI 10.1007/s00044-013-0668-3.
- Faúndez G, Troncoso M, Navarrete P, Figueroa G (2004). Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol 4:19-26.
- Georgakilas V, Kordatos K, Prato M, Guldi DM, Holzinger M, Hirsch A (2002) Organic Functionalization of Carbon Nanotubes. J. Am. Chem. Soc 124: 760-761.
- Hamon MA, Chen J, Hu H, Chen Y, Itkis ME, Rao AM, Eklund PC, Haddon RC (1999) Dissolution of Single-Walled Carbon Nanotubes. Adv Mater 11: 834-840.
- Hu C. Y, Xu Y. J, Duo S. W, Zhang R. F, Li M. S (2009) Non-Covalent Functionalization of Carbon Nanotubes with Surfactants and Polymers. J. Chin. Chem. Soc.56: 234-239.
- Holzinger M, Vostrowsky O, Hirsch A, Hennrich F, Kappes M, Weiss R (2001) Sidewall Functionalization of Carbon Nanotubes. Angew. Chem. Int. Ed. Engl. 40: 4002-4005.
- Karchemski F, Zucker D, Barenholz Y, Regev O, (2012) Carbon nanotubes-liposomes conjugate as a platform for drug delivery into cells. Journal of Controlled Release 160: 339–345.
- Liu S, Hu M, Helen Zeng T, Wu R, Jiang R, Wei J, Wang L, Kong J, Chen Y (2012) Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir . 28: 12364−12372.
- Makarov V, Riabova OB, Yuschenko A, Urlyapova N, Daudova A, Zipfel PF, Mo¨llmannm U, (2006) Synthesis and antileprosy activity of some dialkyldithiocarbamates. Journal of Antimicrobial Chemotherapy. 57 :1134–1138.
- Safari M, Yousefi M, Jenkins H.A , Bikhof Torbati M, Amanzadeh A (2013) Synthesis, spectroscopic characterization, X-ray structure, and in vitro antitumor activities of new triorganotin(IV) complexes with sulfur donor ligand. Med Chem Res. doi:10.1007/s00044-013-0549-9.
- Saha S, Dhanasekaran D, Chandraleka S, Panneerselvam A, (2009) Synthesis, characterization and antimicrobial activity os cobalt metal complex against multi drug resistant bacterial and fungal pathogens . Facta universitatis 7: 73 – 80.
- Sun Y. P, Fu K, Lin Y, Huang W (2002). Functionalized carbon nanotubes: properties and applications. Acc. Chem. Res. 35: 1096-1104.
- Stanila A, Braicu C, Stanila s, M. POP R, (2011) Antibacterial Activity of Copper and Cobalt Amino Acids Complexes. Not Bot Horti Agrobo 39: 124-129.
- Tella A.C, Obaleye J.A (2009) Copper(II) Complexes of 4, 4- Diaminodiphenylsulphone:Synthesis, Characterization and Biological Studies E-Journal of Chemistry. 6: S311-S323.
- Tian Z, Shi Y, Yin M, Shen H, Jia N (2011) Functionalized Multiwalled Carbon Nanotubes-anticancer Drug Carriers: Synthesis, Target-ing Ability and Antitumor activity. Nano Biomed. Eng. 3: 157-162.
- Wadher SJ, Puranik MP, Karande NA, Yeole PJ (2009) Synthesis and Biological Evaluation of Schiff base of Dapsone and their derivative as Antimicrobial agents. International Journal of PharmTech Research.1: 22-33.
- Wu H, Liu G, Wang X, Zhang J, Chen Y, Shi J, Yang H, Hu H, Yang S, ( 2011) Solvothermal synthesis of cobalt ferrite nanoparticles loaded on multiwalled carbon nanotubes for magnetic resonance imaging and drug delivery. Acta Biomaterialia 7: 3496-3504.
- Yousefi M, Safari M, Bikhof Torbati M, Molla Kazemiha V, Sanati Hasan, Amanzadeh A (2012) New mononuclear diorganotin(IV) dithiocarboxylates: synthesis, characterization and study of their cytotoxic activities. Appl. Organometal. chem. 26:438:444.
- Yousefi M, Safari M, Bikhof Torbati M, Amanzadeh A (2014) In vitro anti-proliferative activity of novel hexacoordinated triphenyltin(IV) trifluoroacetate containing a bidentate N-donor ligand. J. Struct. Chem. 55: 101-106.

This work is licensed under a Creative Commons Attribution 4.0 International License.









