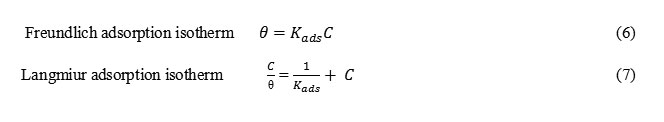Effect of Iodide on the Corrosion Inhibitive Behaviour on Carbon steel by an Azomethine Compound Derived from Anthracene-9(10 H)-one
Shaju K S, Joby Thomas K* And Vinod P. Raphae
Research Division, Department of Chemistry St. Thomas’ College (University of Calicut), Thrissur, Kerala
DOI : http://dx.doi.org/10.13005/ojc/300255
Article Received on :
Article Accepted on :
Article Published : 28 Jun 2014
The inhibition effect of (s)-2-(anthracene-9(10H)-ylideneamino)-5-guanidinopentanoic acid (A9Y5GPA) on carbon steel (CS) in 0.5 M sulphuric acid solution has been investigated using weight loss measurements, electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization studies. The addition of KI to A9Y5GPA enhanced the inhibition efficiency due to synergistic effect. The adsorption of A9Y5GPA and A9Y5GPA+ KI on the carbon steel surface obeys Freundlich and Langmuir adsorption isotherm respectively. The result showed that compound studied was acted as a mixed type inhibitor causing blocking of active sites on the metal. Surface morphology of the carbon steel specimens was evaluated by SEM analysis. Keywords: Carbon Steel, Adsorption, Weight loss, Isotherm, Synergism, Impedance, Polarization.
KEYWORDS:Effect of Iodide; The inhibition;impedance spectroscopy
Download this article as:| Copy the following to cite this article: Shaju K S, Thomas K. J, Raphae V. P. Effect of Iodide on the Corrosion Inhibitive Behaviour on Carbon steel by an Azomethine Compound Derived from Anthracene-9(10 H)-one. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Shaju K S, Thomas K. J, Raphae V. P. Effect of Iodide on the Corrosion Inhibitive Behaviour on Carbon steel by an Azomethine Compound Derived from Anthracene-9(10 H)-one. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=4089 |
Introduction
An effective method employed by corrosion engineers to control the corrosion of metal is the use of certain organic inhibitors1. Organic molecules possessing azomethine linkage (C=N) act as effective potential corrosion inhibitors2-6. The presence of halide ions in solutions has been found to stabilize the adsorption of some organic cations, leading to improved inhibition efficiency7. In continuation of our work8, we report in this paper the corrosion inhibition behaviour and synergism mechanism with I– of a azomethine compound (A9Y5GPA) derived from anthracene-9(10H)-one and (s)-2-amino-5-guanidinopentanoic acid in 0.5M H2SO4 solution on CS at 303 K.
Experimental
A9Y5GPA Solutions
The azomethine compound was synthesized and characterized through their spectral data8. The molecular structure of the compound is shown in the figure 1.
 |
Fig1: Molecular Structure of A9Y5GPA Click here to View Figure |
The aggressive solution of 0.5M H2SO4 was prepared by the dilution of A.R grade 98% of H2SO4 (Merck) with de-ionized water. A9Y5GPA solutions were prepared in the range, 0.2mM-1mM concentrations. 0.2 mM KI solution was also prepared.
Weight Loss Measurements
Weight loss experiments were performed with CS specimens of dimension 1.5x 2x 0.1 cm (Composition: C,0.5%; Mn,0.07%; P,0.02%; S,0.015%; Si,0.02% and rest Fe). The specimens were first polished with different grades of emery papers, rinsed with ethanol and water, respectively. Then dried, weighed and immersed in 50 ml corrosive medium with and without different concentrations of A9Y5GPA and A9Y5GPA + 1ml KI solutions. Weight loss of metal specimens was noted after 24 h. The corrosion rate (ν) and the percentage of inhibition efficiency () were calculated by the following equations9, 10.
Formula 1,2
where W is the weight loss (g) of coupon, S is the total area (cm2) of specimens, t is the time of treatment (24 hrs), ν0 and ν are the corrosion rates of uninhibited and inhibited specimens respectively.
Electrochemical Investigations
The impedance measurements and polarization curves were performed in a three electrode assembly. Saturated calomel electrode (SCE) was used as the reference electrode. Platinum electrode having 1cm2 area was taken as counter electrode. Metal specimens with an exposed area of 1cm2 were used as the working electrode. The EIS experiments were carried out on an Ivium compactstat-e electrochemical system. 100 ml of 0.5M H2SO4 with A9Y5GPA and A9Y5GPA + 2ml KI (no stirring) were taken as the electrolyte and the working area of the metal specimens were exposed to the electrolyte for 1 h prior to the measurement.
EIS measurements were performed at constant potential (OCP) in the frequency range from 1 KHz to 100 mHz with amplitude of 10 mV as excitation signal. The percentage of inhibitions from impedance measurements were calculated using charge transfer resistance values by the following expression11:
Formula3
where Rct and R’ct are the charge transfer resistances of working electrode with and without inhibitor respectively.
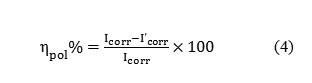
Polarization plots were obtained in the electrode potential range from -100 to +100 mV Vs equilibrium potential (Ecorr) at a scan rate of 1mV/sec. Tafel polarization analysis were done by extrapolating anodic and cathodic curves to obtain corrosion current densities ( Icorr). The percentage of inhibition efficiency (ηpol%) was evaluated from the measured Icorr values using the relation
Formula4
where Icorr and I’corr are the corrosion current densities of the exposed area of the working electrode in the absence and presence of inhibitor. Surface analyses were performed using scanning electron microscope (model Hitachi SU6600).
Results and Discussion
Weight Loss Measurements
The calculated values of inhibition efficiency (ηW %) and surface coverage (θ) at 300C from the losses of weight of CS after immersing in solutions of 0.5 M H2SO4 containing different concentrations of the A9Y5GPA are given in table 1.
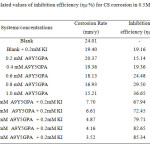 |
Table1: Calculated values of inhibition efficiency (ηW %) for CS 0.5M H2SO4 corrosion in Click here to View table |
From table 1 it is clear that the inhibition efficiency increases with increasing the concentration of the compound. Various concentrations of A9Y5GPA alone show very low inhibition values. This behaviour indicates that the A9Y5GPA can act as a moderate inhibitor for CS corrosion in 0.5M H2SO4 solution. However the A95GPA + KI system shows high inhibition values. The inhibitive action of the used compound is attributed to the adsorption of molecules on CS surface, forming a barrier between the metal surface and the corrosive environment.
Synergistic Effect
From table 1 it is also evident that ηW % for KI in combination with A9Y5GPA is higher than the sum of ηW % for single KI and single A9Y5GPA in all investigations, which is synergism in nature. Aramaki and Hackerman12 calculated the synergism parameter Sθ using the following equation
Formula5
where ; θ1= surface coverage by anion; θ2= surface coverage by cation; θ’1+2 = measured surface coverage by both anion and cation. Sθ approaches unity when there are no interactions between the inhibitor compounds, while Sθ > 1 points to a synergistic effect; in the case of Sθ <1, the antagonistic interaction prevails. The values of the synergism parameter for the various concentrations of A9Y5GPA studied from the gravimetric analysis are presented in table 2.
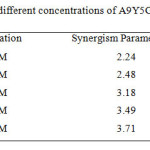 |
Table 2: Synergism parameter (Sθ) for different concentrations of A9Y5GPA in combination with 0.2mM KI Click here to View table |
All values shown in this table are greater than unity. This is an indication for the enhanced inhibition efficiency obtained by the addition of iodide ions to A9Y5GPA is synergistic in nature13.
Adsorption Studies
The best description of the adsorption behavior of A9Y5GPA and A9Y5GPA + KI on CS specimens in 0.5M H2SO4 was Freundlich and Langmiur adsorption isotherms respectively. These models are expressed as14 Freundlich adsorption isotherm Langmiur adsorption isotherm
Formula6,7
where C is the concentration of the inhibitor, θ is the fractional surface coverage and Kads is the adsorption equilibrium constant. Figs. 2 and 3 represent the adsorption plots of A9Y5GPA and A9Y5GPA+ KI obtained by the weight loss measurements of CS steel specimens in 0.5M H2SO4 at 300C for 24 h respectively.
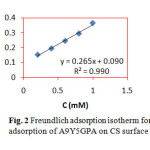 |
Fig2: Freundlich adsorption isotherm for adsorption of A9Y5GPA on CS surface Click here to View Figure |
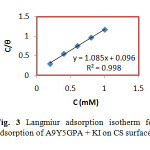 |
Fig3:Langmiur adsorption isotherm for adsorption of A9Y5GPA + KI on CS surface Click here to View Figure |
The adsorption equilibrium constant Kads is related to the standard free energy of adsorption ∆G0ads, by
ΔG0 ads = _RTln (55.5 Kads) (8)
Where 55.5 is the molar concentration of water, R is the universal gas constant and T is the temperature in Kelvin15. In the present study, A9Y5GPA and A9Y5GPA + KI molecules showed ∆G0ads -24.2 KJ and -27.5 KJ respectively, suggesting that the adsorption of inhibitor involves both electrostatic and chemical interactions.
Electrochemical Investigations
Figs.4 and 5 represent the Nyquist plots of CS specimens in 0.5M H2SO4 in the presence of various concentrations of A9Y5GPA and A9Y5GPA + KI respectively. It is evident from the plots that the impedance response of metal specimens has marked difference in the presence and absence of the KI with A9Y5GPA.
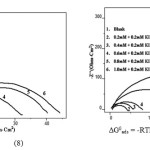 |
Fig4: Nyquist plots for CS specimens in A9Y5GPA solutions |
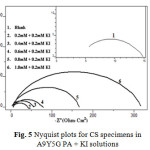 |
Fig5: Nyquist plots for CS specimens in A9Y5G PA + KI solutions
|
Impedance behaviour can be well explained by pure electric models that could verify and enable to calculate numerical values corresponding to the physical and chemical properties of electrochemical system under examination16. The simple equivalent circuit that fit to many electrochemical systems composed of a double layer capacitance (Cdl), solution resistance (Rs) and charge transfer resistance (Rct)17, 18. The impedance parameters are tabulated in the table 3 (3a and 3b).
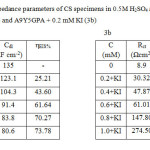 |
Table3: Electrochemical impedance parameters of CS specimens in 0.5M H2SO4 at 300C in the absence and presence of A9Y5GPA (3a) and A9Y5GPA + 0.2 mM KI (3b) Click here to View table |
From tables 3a and 3b it is clear that Rct values are increased with increasing A9Y5GPA concentration. The capacitance values Cdl decreases with A9Y5GPA concentration and this decrease in Cdl is enhanced upon addition of I− ions to the corrosive environment. These results suggest that the A9Y5GPA molecules function by adsorption at the metal/solution interface19 and this adsorption is reinforced by I− ions. The ηEIS% data reveal that the corrosion inhibition capacity of A9Y5GPA is markedly enhanced by the addition of KI.
Potentiodynamic polarization curves for A9Y5GPA in 0.5M H2SO4 at 30 0C for CS specimens in the presence of various concentrations of A9Y5GPA and A9Y5GPA + KI are shown in Fig.6 and Fig.7 respectively.
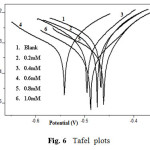 |
Fig6: Tafel plots |
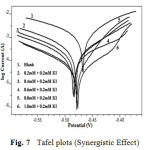 |
Fig7: Tafel plots (Synergistic Effect) |
Table 4 Potentiodynamic polarization parameters of CS specimens in 0.5M H2SO4 at 300C
|
A9Y5GPA Alone |
A9Y5GPA + 2ml KI |
||||||||||
|
C (mM) |
Ecorr (mV/SCE) |
Icorr (mA/ cm2) |
ba (mV/ dec) |
-bc (mV/ dec) |
ηpol% |
C (mM) |
E orr (mV/SCE) |
Icorr (mA/ cm2) |
ba (mV/ dec) |
-bc (mV/ dec) |
ηpol% |
|
0 |
-467 |
1.170 |
0.082 |
0.124 |
– |
0 |
-467 |
1.170 |
0.082 |
0.124 |
– |
|
0.2 |
-475 |
0.831 |
0.072 |
0.126 |
28.97 |
0.2 |
-472 |
0.2297 |
0.072 |
0.100 |
80.37 |
|
0.4 |
-494 |
0.7102 |
0.071 |
0.102 |
39.30 |
0.4 |
-475 |
0.1495 |
0.058 |
0.101 |
87.22 |
|
0.6 |
-541 |
0.6304 |
0.080 |
0.119 |
46.12 |
0.6 |
-482 |
0.1261 |
0.099 |
0.100 |
89.22 |
|
0.8 |
-461 |
0.4316 |
0.063 |
0.123 |
63.11 |
0.8 |
-475 |
0.0545 |
0.037 |
0.097 |
95.34 |
|
1.0 |
-488 |
0.2656 |
0.065 |
0.105 |
77.30 |
1.0 |
-466 |
0.0335 |
0.053 |
0.089 |
97.14 |
Polarization parameters like corrosion current densities (Icorr), corrosion potential (Ecorr), cathodic Tafel slope (bc), anodic Tafel slope (ba), and inhibition efficiency (η pol%) for CS specimens are listed in table 4 . The data show that, addition of the A9Y5GPA to acid media affected both the cathodic and anodic parts of the curves. Addition of I– ions to A9Y5GPA – H2SO4 systems results in marked decrease in the corrosion current density (Icorr). The maximum shift of Ecorr is 74mV, suggesting that A9Y5GPA acts as a mixed type inhibitor for CS specimens in 0.5M H2SO4. From the values it is clear that the inhibition efficiency of A9Y5GPA alone is increased in presence of KI. These results also confirm the existence of strong synergism between A9Y5GPA and KI in the corrosion inhibition of CS in these solutions.
SEM Studies
The surface morphology of carbon steel surface was evaluated by scanning electron microscopy (SEM). Figures 8 (A, B, C and D) show the scanning electron micrographs of the bare carbon steel surface, CS specimens in H2SO4, with A9Y5GPA and with A9Y5GPA+ KI in H2SO4 medium for 24 h respectively. The morphology revealed that in the absence of A9Y5GPA, the surface is highly corroded. However in the presence of A9Y5GPA the rate of corrosion is suppressed and the SEM image D shows that inhibition action enhanced by forming a protective layer on the surface that prevents the attack of acid on the corroding metal CS.
 |
Fig8: Scanning Electron Micrographs of carbon steel samples: A) Polished fresh specimens B) Immersed in 0.5M H2SO4 solution C) Immersed in 0.5M H2SO4 solution with 1mM A9Y5GPA C) Immersed in 0.5M H2SO4 solution with 1mM A9Y5GPA+ 1ml KI Click here to View Figure |
Mechanism and Explanation for synergism
The synergistic inhibition brought about by the combination of A9Y5GPA and iodide ions for the corrosion of CS in 0.5 M H2SO4 can be explained on the basis that halide ions have a greater tendency to be adsorbed on the surface in attraction with organic cations.
The protonated Schiff base (A9Y5GPA+) is adsorbed by coulombic attraction at the steel surface, where iodide ions are already adsorbed by chemisorptions. Greater surface coverage from the stabilization of adsorbed iodide ions by means of electrostatic interaction with A9Y5GPA+ facilitates corrosion inhibition synergism.
Conclusions
- Inhibition efficiency increases with increase in concentration of inhibitor.
- The addition of iodide ions to A9Y5GPA enhanced the inhibition efficiency due to synergistic effect.
- The adsorption of A9Y5GPA alone and in combination with iodide ions obeys Freundlich and Langmiur adsorption isotherms respectively.
- The thermodynamic parameters calculated from the adsorption isotherms showed that both physisorption and chemisorptions are involved in the inhibition process.
Reference
- James, O; Oforka, N. C; Abiola, O.K; Int. J. Electrochem. Sci. 2007, 2, 278-284
- Sethi, T. A.; Chaturvedi, R. K; Upadyay; Marthur, S. P; J. Chil. Chem. Soci. 2007, 52, 1206-1213
- Srivastva, K. P; Kumar, A; Singh, R; J. Chem. Pharm. Res. 2010, 2, 68-77
- Ade, S. B; Deshpande, M. N; Kolhatkar, D. G; J. Chem. Pharm. Res. 2012, 4, 1033-1035
- Vinod, P. R; Joby, T.K; Shaju, K.S; Aby, P; Res. Chem. Intermed. 2013, DOI 10.1007/s11164-013-1122-3
- Aby, P; Joby, T.K; Vinod, P. R; Shaju, K.S; Oriental J. Chem. 2012, 28, 1501-1507
- Shaju, K.S; Joby, T.K; Vinod, P.R; Aby, P; Hindawi Publishing Corporation, ISRN Corrosion. 2012, http://dx.doi.org/10.5402/2012/4258
- Shaju, K. S; Joby, T.K; Vinod, P. R; Aby, P; Hindawi Publishing Corporation, ISRN Electrochemistry. 2013, http://dx.doi.org/10.1155/2013/820548
- Deng, S; Li, X; Fu, H; Corros. Sci. 2011, 53, 3704-3711
- Emregul, K.C; Atakol,O; Mater. Chem. Phys. 2004, 83, 373-379
- Ashassi-Sorkhabi, H; Shaabani, B; Seifzadeh, D; Electrochim. Acta. 2005, 50, 3446-3452
- Aramaki, K; Hackerman, M; J. Electrochem. Soc. 1969, 116, 568-574
- Caliskan, N; Bilgic, S; Appl. Surf. Sci. 2000,153, 128-133
- Bouklah, M; Hammouti, B; Lagrenée, M; Bentiss, F; Corros. Sci. 2006, 48, 2831-2842
- Cano, E; Polo, J.L; Iglesia, A.L.A; Bastidas, J.M; Adsorption. 2004, 10, 219-225
- Priya, A.R.S; Muralidharam,V.S; Subramannia, A; Corrosion. 2008, 64, 541-552
- Azhar, M.E; Mernari, B; Traisnel, M; Bentiss, F; Lagrenée, M; Corros. Sci. 2001, 43, 2229-2238
- Yurt, A; Balaban, A; Kandemir, S.U; Bereket, G; Erk, B; Mater. Chem. Phys. 2004, 85, 420-426
- MaCafferty, M; Hackerman, N; J. Electrochem. Soc.1972, 119,146-154

This work is licensed under a Creative Commons Attribution 4.0 International License.