Corrosion Inhibition of Aluminum in 0.5 M HCl by Garlic aqueous extract
Saedah R. Al-Mhyawi1
King Abd El-Aziz university, Girls College of Education, Chemistry Department, Jeddah, KSA.
DOI : http://dx.doi.org/10.13005/ojc/300218
Article Received on :
Article Accepted on :
Article Published : 09 Jun 2014
The inhibition efficiency of extract of Garlic on aluminium in hydrochloric acid solutions has been evaluated by weight loss techniques. Values of inhibition efficiency obtained are dependent upon the concentration of inhibitor and temperature. Generally, inhibition was found to increase with inhibitor concentration, half-life, activation energy but decrease with temperature and first-order rate constant at the temperatures studied. Physical adsorption mechanism has been proposed for the inhibition and Langmuir , Temkin adsorption isotherm was obeyed. Garlic is an inhibitor of aluminium corrosion in 0.5 M hydrochloric acid solution.The values of standard free energy of adsorption suggest that the adsorption of inhibitor on aluminium surface occurred by physisorption mechanism. the negative sign of the Free Energy of adsorption indicates that the adsorption of the inhibitors on the aluminum surface was a spontaneous process.the negative values of enthalpy of adsorption (ΔH) suggest that the chemical reaction involved in the adsorption of the inhibitors on the metal surface is an exothermic process, hence increase in the reaction temperature of the medium will decrease the inhibition efficiency.
KEYWORDS:Corossion; inhibition; Langmur; Temkin adsorption isotherm; half-life; activation energy; free energy.
Download this article as:| Copy the following to cite this article: Al-Mhyawi S. R. Corrosion Inhibition of Aluminum in 0.5 M HCl by Garlic aqueous extract. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Al-Mhyawi S. R. Corrosion Inhibition of Aluminum in 0.5 M HCl by Garlic aqueous extract. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3760 |
Introduction
Corrosion of metals is mainly due to an irreversible oxidation–reduction reaction, where an oxidizing agent in an environment attacks the metal. An electrochemical reaction is a chemical transformation that implies charge transport at the interface from a metallic conductor (electrode) to an ionic conductor (electrolyte). These are dependent on the thermodynamic and physical properties of the electrode and the activities of the different species in solution at the interface. The properties of the interface are dependent on temperature variation, bulk solution properties, convection, diffusion, and so on. In addition,metallurgical and mechanical properties of the electrode and microbiological organisms in solution or at the interface can cause corrosion alone or increase corrosion rates by a synergetic effect with electrochemical corrosion. In other limited conditions of corrosion,chemical reactions, such as the dissolution in liquid metals and some solvent media and metal–gas free interface reactions, can cause corrosion alone or assist an electrochemical reaction 1.
Aluminum is actually a very active metal, meaning that its nature is to oxidize very quickly. While a weakness for most metals, this quality is actually the key to its ability to resist corrosion. When oxygen is present (in the air, soil, or water), aluminum instantly reacts to form aluminum oxide. This aluminum oxide layer is chemically bound to the surface, and it seals the core aluminum from any further reaction. This is quite different from oxidation (corrosion) in steel, where bnrust puffs up and flakes off, constantly exposing new metal to corrosion. Aluminum’s oxide film is tenacious, hard, and instantly self-renewing. According to the US Army Corps of Engineers, “Aluminum has excellent corrosion resistance in a wide range of water and soil conditions because of the tough oxide film that forms on its surface. Although aluminum is an active metal in the galvanic series, this film affords excellent protection except in several special cases 2 .
Aluminum and its alloys are widely used in many industries such as reaction vessels, pipes, machinery and chemical batteries because of their advantages. Hydrochloric acid (HCl) solutions are used for pickling, chemical and electrochemical etching of aluminum. It is very important to add corrosion inhibitors to prevent metal dissolution and minimize acid consumption 3.
Aluminum is an important metal in many industries owing to its many excellent characteristics especially its good electrical and thermal conductivities, low density, high ductility, low cost and availability for the fabrication and construction industries. It is widely used as a material for automobiles, aviation, household appliances, containers, electronic devices, pipes, machinery, and chemical batteries 4.
Aluminum is an important material for use in many applications, such as for automobiles, aviation, household appliances, containers, and electronic devices 4, owing to its many favorable characteristics including its good electrical and thermal conductivities, low density, high ductility,and good corrosion resistance. The corrosion resistance of aluminum arises from its ability to form a natural oxide film on its surface in a wide variety of media 5, , and it has been reported that the oxide film can readily undergo corrosion reactions in chloride environments 6,7. For this reason,the corrosion mechanism of aluminum in chloride solutions has been investigated in a number of studies 8,9.
Among many methods of corrosion control and prevention, the use of organic inhibitors is the most frequently used one. Organic inhibitors are mostly used to protect aluminium and alloys against corrosion in aggressive electrolytes because they adsorb on the surface by acting as a protective film on anodic and cathodic areas simultaneously 10. Most of organic compounds contain polar groups such as nitrogen, sulfur,and oxygen 11. The inhibition of organic compounds is based on the adsorption ability of their molecules. The inhibitor molecules are bonded on the metal surface as chemisorptions, by physical adsorption, or by complexation with the polar groups acting as the reactive centers in the molecules 12.
Adsorption characteristics of these inhibitors depend on a few factors; such as the surface charge of the metal, the chemical structure of organic inhibitors,the distribution of charge in the molecule, the type of aggressive electrolyte, and the type of interaction between the organic molecules and the metallic surface 10,12.
Green Inhibitors
Green corrosion inhibitors are biodegradable and do not contain heavy metals or other toxic compounds. Some research groups have reported the successful use of naturally occurring substances to inhibit the corrosion of metals in acidic and alkaline environment. Delonix regia extracts inhibited the corrosion of aluminum in hydrochloric acid solutions 13, rosemary leaves were studied as corrosion inhibitor for the Al + 2.5Mg alloy in a 3% NaCl solution at 25◦C 14, and El-Etre investigated natural honey as a corrosion inhibitor for copper 15 and investigated opuntia extract on aluminum 16.
The inhibition efficiency of acetone extract of red onion skin on aluminium in hydrochloric acid solutions has been evaluated by weight loss technique17 . Values of inhibition efficiency obtained are dependent upon the concentration of inhibitor and temperature.
Generally, inhibition was found to increase with inhibitor concentration, half-life, activation energy but decrease with temperature and first-order rate constant at the temperatures studied. Physical adsorption mechanism has been proposed for the inhibition and Langmuir adsorption isotherm was obeyed. The compound responsiblefor the inhibitory action of red onion skin is Quercetin. Red onion skin is an inhibitor of aluminium corrosion in 2 M hydrochloric acid solution.
Abiola and Tobun18 studied the ability of cocos nucifera L.water(CW) as non-toxic corrosion inhibitor for acid corrosion of aluminum in 0.5 mol/L HCl using chemical technique.CW shows significant inhibition as corrosion inhibitors,with 93% efficiency at the highest concentration of the inhibitor.The inhibitive action is attributed to ythe adsorption of the inhibitor molecules on metal surface dfollowing Langmuir adsorption isotherm.
Li and Deng studied 19 the inhibition effect of Dendrocalamus brandisii leaves extract (DBLE) on the corrosion of aluminum in HCl, H3PO4 solutions was studied by weight loss, potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and scanning electron microscopy (SEM) methods. Effects of temperature, immersion time and acid concentration on corrosion inhibition were fully discussed. The results show that DBLE is a good inhibitor in 1.0 M HCl, while a moderate inhibitor in H3PO4. The adsorption of DBLE on aluminum surface obeys Langmuir isotherm in both acids. DBLE acts as a cathodic inhibitor in HCl, while a mixed-type inhibitor in H3PO4.
The corrosion behaviour of Aluminium and copper exposed to HCl solution and their inhibition in HCl containing 0.0644 – 1.288 g/L Ziziphus mauritiana Fruit Extract used as inhibitor was studied at room temperature using weight loss method 20. From weight loss data it was observed that the Inhibition efficiency increases with increase in concentration of the inhibitor reaching a maximum of 76.8% at room temperature for aluminium and 88.58% at room temperature for copper at 1.288 g/L concentration of EEZmF. Corrosion rate was also found to decrease in the presence of inhibitor compared to the free acid solution. The inhibitor,Ziziphus mauritiana was found to obey Langmuir adsorption isotherm for both the metals. Ziziphus mauritiana is a better corrosion inhibitor for copper than aluminium. Surface analysis (FT-IR) was also carried out to establish the mechanism of corrosion inhibitor on aluminium and copper corrosion in hydrochloric acid medium.
The aim of this study: was to investigate the inhibition effect of Garlic as a cheap, raw and non-toxic corrosion inhibitor on Aluminum corrosion in hydrochloric acid solution.
Experimental
Specimens and Surface Pretreatment
Acommercial specimen of Al with chemical composition in table 1 was used.
Table1: Percentage composition of the studied specimen.
|
Al |
Ti |
Zn |
Cr |
Mg |
Mn |
Cu |
Fe |
Si |
|
Remainder |
0.03 |
0.04 |
0.04 |
0.66 |
0.40 |
0.05 |
0.25 |
0.95 |
having dimension R =1.37 L=5.38 cm with exposed area of 26.090417 cm². Befor all measurements,the specimen was mechanically polished.This was achieved by using a series of emery papers with different grade (320-600-1200), starting with a coarse one and proceeding in steps to fine grades. After this process the specimen was washed with double-distilled water, degreased with aceton and finally , dried with a stream of air .The specimen was then weighed and immersed in the test solution.
preparation of extract (inhibitor) and Test Solution
The aggressive of 0.5 M HCl was prepared by dilution of AR grade(%) HCl with distilled water.
The inhibitor used was acetone extract of Garlic (allium) skin. The skin of Garlic (allium) bulb was obtained locally from Panda market,jeddah. The Garlic (allium) skin was boiled with 50 ml acetone and 50ml water mixture. The resulting Garlic (allium) solution was heated to dryness; the powder obtained was then scrapped out and stored in a sample bottle. Six different concentrations (0.0025g/dm3,0.0050 g/dm3,0.0075g/dm3, 0.0100g/dm3, 0.0125g/dm3 and 0.0250g/dm3) of the extract were prepared with 0.5 M hydrochloric acid solution and were used for all measurement .
 |
Figure 1 A Click here to View Figure |
Methods
The corrosion rate of Al in 0.5 M HCl followed by using chemical methods . This method is done in, weight loss method(WL).
Weight loss study using the Garlic (allium) acetone extract
Experiments were conducted for the immersion times 30,60,90,120,150,180,210 mins. Preweighted aluminium specimens were suspended for different immersion periods in 0.5 HCl with and without the inhibitor in different concentrations ranging from concentrations (0.0025, 0.0050, 0.0075, 0.0100, 0.0125 and 0.0250 g/dm3) of the extract into seven separate beakers maintained at 303, 313and 323K. .After the specified time the coupons were removed from test solution, thoroughly washed with double distilled water, dried well and then weighted 21,22. The percentage of inhibitor efficiency (IE%) for various concentrations of inhibitor were calculated as 23
%IE = [ (ΔMu-ΔMi) / ΔMu] x 100 (1)
Where ΔMu is mass loss without inhibitor and Δ Mi is mass loss with inhibitor.
Results And Discussion
Effect of Reaction Conditions on Corrosion Rate and Inhibition Efficiency of Garlic Extract
Effect of inhibitor Concentration (Gravimetric results)
The weight losses (gravimetric measurements) for the Aluminium in 0.5 M HCL containing different concentrations of the Garlic extracts as a function of time are presented in Figures. 1,2,3. The results show that weight losses increase with increase in time but decrease with increase in concentration of Garlic extracts.
As shown in Figures 1,2,3, the inhibition efficiency of the extracts on the mild steel increases with increasing concentration of the plant extracts. This is expected because as the concentration of the plant extracts increases, the fraction of the surface covered by the adsorbed molecule also increases which results into an increase in the inhibition efficiency. The inhibition efficiency increases progressively as the concentration of the extracts increases However, the inhibition efficiency decreases with increase in the temperature of the reaction medium.
At 313 K, as the concentration of Garlic skin extract increases from 0.0025 to 0.0250 g/dm3, the weight losses of the aluminium coupons reduce as shown in Figure 2.This shows us that Garlic skin extract is still effective in inhibiting the corrosion of aluminium at 313 K. The weight loss of the aluminium coupons still reduced with increasing extract concentration as seen in Fig. 3. This depicts that, even at 323K, Garlic skin extract inhibits the corrosion of aluminium in hydrochloric acid solution.
The decrease is due to the inhibitive effects of Garlic extracts and these effects increase with increase in Garlic extracts concentration. This behavior indicates that the Garlic extracts is strongly inhibit the corrosion of the aluminium and the degree of inhibition is concentration dependent.
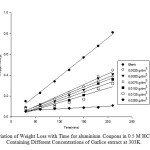 |
Fig1:Variation of Weight Loss with Time for aluminium Coupons in 0.5 M HCl Solution Containing Different Concentrations of Garlice extract at 303. Click here to View Figure |
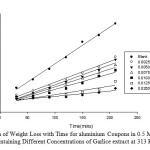 |
Fig2: Variation of Weight Loss with Time for aluminium Coupons in 0.5 M HCl Solution Containing Different Concentrations of Garlice extract at 313 K. Click here to View Figure |
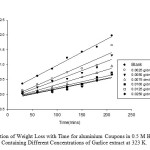 |
Fig 3: Variation of Weight Loss with Time for aluminium Coupons in 0.5 M HCl Solution Containing Different Concentrations of Garlice extract at 323 K. Click here to View Figure |
There is a progressive increase in weight loss as the temperature is increased from 303K to 323K (Figures 1,2,3). This signifies that the dissolution of the metals increased at higher temperatures. This observation is attributed to the general rule guiding the rate of chemical reaction, which says that chemical reaction increases with increasing temperatures. Also an increased temperature favors the formation of activated molecules, which may be doubled in number, with 10oC rise in temperature,thereby increasing the reaction rate. This is because the reactant molecules gain more energy and are able to overcome the energy barrier more rapidly24. An increase in temperature may also increase the solubility of the protective films on the metals, thus increasing the susceptibility of the metal to corrosion 25. The solubility of oxygen gas decreases with increase in temperature.
Thus oxygen concentration is expected to be more at higher temperature which in this case is higher at 323K than at 313 and 303K. The presence of high concentration of oxygen thereby causes the metal to corrode faster. Also for solids, solubility generally increases with increasing temperature. This explains why the protective film which is solid becomes more soluble as the temperature is increased.
Effect of Temperature on Inhibition Efficiency
The effect of temperature on the inhibition efficiency for aluminium in 0.5 M HCl solution in the absence and presence of different concentration of Garlice extract at temperature ranging from 303K to 323 K was obtained by weight loss measurements is displayed graphically in Figure 4.The inhibition efficiencies are found to decrease with increasing the solution temperature from 303 to 323 K.This behavior can be interpreted on the basic that relatively high temperature the increase in temperature results in the desorption of the inhibitor molecules from the surface of aluminium26. Previous investigators showed that the corrosion rate increases with increase in temperature 27, which results into a decrease in the inhibition efficiency, Therefore, decreasing the reaction temperature favours the inhibition efficiency of acid extracts of Garlice extract on aluminium in hydrochloric acid.
Figure 4 also portrays an increase in inhibition efficiency of Garlice extract as the concentration of the extract increases in the acid solution.
However,aluminium corrosion rate in absence and presence of the Garlice extract obeys Arrhenius type reaction 28.
1npwl = 1nA – Ea/ RT——-(2)
where rWL is the corrosion rate obtained from WL measurements,Ea is the apparent activation energy,R is the molar gas constant,T is the absolute temperature and A is the frequency factor
In order to calculate the apparent activation energy of aluminium corrosion in the absence and presence of inhibitor,a plot of lnrWL vs.T-1was done as shown in Figure 5.The slope (Ea/RT) of the straight lines was used to calculate the apparent activation energy while the intercept(ln A) was used to calculate the frequency factor.Table 2 gives the calculated kinetic parameters.
As was shown in Table 2,the activation energy of aluminium corrosion in the inhibited solution using the Garlice extract was endothermic and has greater than that of the uninhibited solution,suggesting that higher energy barrier for the corrosion process in the inhibited solution associated with physical adsorption or weak chemical bonding between the inhibitor species and Garlice aluminium surface29.
Table 2: Activation Energy values with the various concentrations of the extract
|
Concentration of Extract (g.dm3) |
Activation energy KJ mol-1 |
Average Activation energy KJ mol-1 |
||
|
303K-313K |
313K-323K |
303K-313K |
313K-323K |
|
|
Blank |
46.33 |
35.11 |
50.45 |
38.57 |
|
0.0025 |
47.69 |
36.68 |
||
|
0.0050 |
49.03 |
37.57 |
||
|
0.0075 |
49.73 |
37.61 |
||
|
0.0100 |
49.78 |
37.71 |
||
|
0.0125 |
54.89 |
37.96 |
||
|
0.0250 |
55.73 |
47.38 |
||
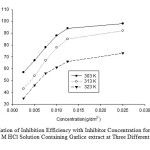 |
Fig4: Variation of Inhibition Efficiency with Inhibitor Concentration for aluminium Coupons in 0.5 M HCl Solution Containing Garlice extract at Three Different Temperatures. Click here to View Figure |
 |
Fig5: Arrhenius plots for aluminium corrosion in 0.5 M HCl in absence and presence of different concentration of Garlice extract at 120 mint Click here to View Figure |
Adsorption Parameters
Adsorption isotherms are very important in determining the mechanism of organo-electrochemical reaction. The inhibition of the corrosion of aluminum in 0.5M HCl medium with addition of different concentrations of the extract can be explained by the adsorption of the components of the plant extract on the metal surface.
The principal step in the accomplishment of inhibitiors action in acid solution is usually established to be the adsorption on the metal surface.This leads to the assumption that the corrosion reactions are prohibited from occurring over the area(or active sites) of the metal surface enclosed by adsorbed inhibitior species,whereas these corrosion reactions take place on the inhibitor-free region30 .
The surface coverage(θ) data are very useful on discussing the adsorption characteristics.When the fraction of surface covered is determined as a function of the concentraction at constant remperature,adsorption isotherm could be evaluated at equilibrium condition.Attemts were made to fit the θ values to various isotherm including Langmuir,Temkin,Frumkin,ElAwady,Freundlich and Flory-Huggins ect.The best fit was obtained with Langmuir isotherm as suggested by the plot between C/θ and C (as shown in Figure 6) and the linear correlation coefficient of the fitted data was close to 1,indicating that the adsorption of the inhibitor molecules obey the Langmuir’s adsorption isotherm as expressed as
Cθ-1=1/Kads.+Cinh. (3)
Where Cinh. is the inhibitor concentration and Kads.is the equilibrium constant for adsorption/desorption process of the inhibitor molecules on the metal surface. Kads. Values were calculated from the intercept of the plot for adsorption process and related with the free energy change of adsorption ,∆G0ads,by the following equation
,∆G0ads= -RT ln(kads.Cwater) (4)
The Langmuir adsorption parameters and the values of ,∆G0ads in thepresenceofGarlice extract are given in table 3.Inspection of Table 3 reveals that the negative values of ,∆G0ads point to the strength of the adsorbed layer on the Aluminum surface and naturalness of the adsorption process with the formation of an adsorbed film on the Aluminium surface 31 .
The plot of θ vs log C was linear for Garlice extract (see Figute 7). The plots support the assertion that the mechanism of corrosion inhibition is due to the formation and maintenance of a protective film on the metalsurface and that the additive covers both the anodic and cathodic sites through uniform adsorption 32. The fit of the experimental data to these isotherms provide evidence for the role of adsorption in the observed inhibitive effect of the Garlice extract.
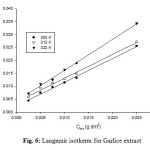 |
Fig6: Langmuir isotherm for Garlice extract: Click here to View Figure |
 |
Fig7: Temkin isotherm for Garlice extract Click here to View Figure |
Table 3:Adsorption Equilibrum constant (Kads) at different Temperatures
|
Temperature (K) |
K ads. |
R2 |
|
303 |
0.422 |
0.9964 |
|
313 |
0.256 |
0.9944 |
|
322 |
0.237 |
0.9977 |
Thermodynamic Study
Determination of Enthalpy and Entropy
Thermodynamic parameters such as enthalpy (ΔH) and entropy (ΔS) of activation of corrosion process may be evaluated from the effect of temperature. The enthalpy and entropy of activation of corrosion process was calculated from the equation
Log CR/T = log (R/nh ) + ∆S/2.303R-∆H/2.303RT……………..(5)
Where „CR‟ is the corrosion rate, „T‟ is the absolute temperature , „R‟ is the molar gas constant, „n‟ is Avogadro‟s constant, and „h‟ is the Planck‟s constant. A plot of log CR/T vs 1/T is a straight line graph (see Figure 8) with a slope of (-∆H/2.303R ) and an intercept of (log (log (R/nh ) + ∆S/2.303R ). From the slope and the intercept, ΔH and ΔS were calculated as reported by Abiola et al 13,33.
The results presented in Table 4 show that the enthalpy of activation values were all negative for Garlice extract which reflects the exothermic nature of the aluminum dissolution process. Also, the entropies of activation were negative indicating that the activation complex represents association steps and that the reaction was spontaneous and feasible. These results were in excellent agreement with the previous study 34.
Free Energy of Adsorption
The free energy of adsorption values, ΔG°ads,were obtained by using equation (8) and the values obtained are presemted in Table 5.
ΔG°ads= -2.303RT log (55.5Kads) ………………………………… (6)
Results obtained indicate that the values of ΔG°ads are negative in all cases, showing that the reaction is spontaneous 33 and that the Garlice extracts are strongly adsorbed on the aluminum surface by physical adsorption. Previous investigators showed that the values of ΔG°ads up to -20 kJmol-1 are consistent with electrostatic interaction between charged molecules and a charged metal (which indicates physisorption), while those more negative than -20 KJ mol-1 involve charge sharing or transfer from the inhibitor molecules to the metal surface to form a co-ordinate type of bond (which indicates chemisorption) . This observation also supports the earlier assertion that a physical adsorption is proposed as a result of electrostatic attraction between charged metal surface and charged species in the bulk of the solution. The ΔG values which are less than 10.5 kJ/mol for various inhibitor concentrations (Table 5) revealed the decreased rate of corrosion reaction which is also substantiated by other studies 13,33.
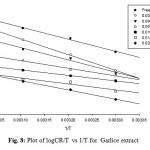 |
Fig8: Plot of logCR/T vs 1/T for Garlice extract Click here to View Figure |
Kinetic Study
Determination of Rate Constant
The corrosion reaction is a heterogeneous one, composed of anodic and cathodic reactions with the same or different rate. It is on this basis that kinetic analysis of the data is considered necessary.
In this present study, the initial weight of aluminum coupon at time, t is designated Wi, the weight loss is ∆W and the weight change at time t, (Wi – ∆W). The plots of log (Wi – ∆W) against time (min) at 303 K and other temperatures studied, showed a linear variation which confirms a first order reaction kinetics with respect to the corrosion of aluminium corrosion in 0.5 M HCl solutions in the presence of the Garlice extract (Figure 9). The rate constant, half-life time and activation energy were calculated as shown in our earlier report 35. There is a
Table 4:Enthalpy and Entropy values of the reaction with various concentrations of the extracts Concentration of Extract (g.dm3) ΔH (KJ mol-1) ΔS (J mol-1K-1)
|
Concentration of extract (g.dm3) |
-∆H0(kJ mol-1) |
– ∆S0( J .mol-1.K-1) |
|
Blank |
47.15 |
232.60 |
|
0.0025 |
36.77 |
205.19 |
|
0.0050 |
36.21 |
183.51 |
|
0.0075 |
27.11 |
174.36 |
|
0.0100 |
22.82 |
135.37 |
|
0.0125 |
16.48 |
132.00 |
|
0.0250 |
8.01 |
101.61 |
Table 5: The Free energy of Adsorptions at various Temperatures
|
Temperature(K) |
-∆G0(kJ mol-1 |
|
303 |
7.945 |
|
312 |
6.907 |
|
322 |
6.899 |
general decrease in the rate constants from 303K – 323K with increasing concentrations of the red onion skin extract (Table 6).
Determination of Half Life
The values of half-life, t1/2, were calculated using the equation:
t1/2 =0.693/k
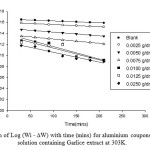 |
Fig9: Variation of Log (Wi – ∆W) with time (mins) for aluminium coupons in 0.5M HCl solution containing Garlice extract at 303K. Click here to View Figure |
The increase in half-life (t1/2) shown when the the Garlice extract is present further supports the inhibition of aluminium in 0.5 M HCl by the additives. The increase in half life indicates more protection of the metals by the the Garlice extract. On the basis of the experimentally determined activation energy value (50.45 kJ mol-1 at 303 – 313 K), the additive is physically adsorbed on the coupons.Therefore, it is probable that a multilayer protective coverage on the entire aluminium surface was obtained 13.
Phytochemical Constituents
Garlic contains at least 33 sulfur compounds like aliin, allicin, ajoene, allylpropl, diallyl, trisulfide, sallylcysteine, vinyldithiines, S-allylmercaptocystein, and others. Besides sulfure compounds garlic contains 17 amino acids and their glycosides, arginine and others. Minerals such as selenium and enzymes like allinase, peroxidases, myrosinase, and others. Garlic contains a higher concentration of sulfur compounds than any other Allium species. The structure of the main representative compounds in Garlic are shown in Figure 10.
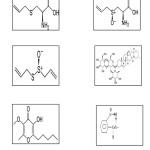 |
Fig10: The structure of some compounds present in Garlic plant Click here to View Figure |
Table 6:Kinetic data for aluminium in 2M HCl containing red onion skin extract from weight loss measurement.
|
The studied solution |
Rate constant K(min-1)X10-5
|
Half-Life t1/2(mins)x104 |
Activation energy KJ mol-1 |
Average Activation energy KJ mol-1 |
||||||
|
303K |
313K |
323K |
303K |
313K |
323K |
303K-313K |
313K-323K |
303K-313K |
313K-323K |
|
|
Blank |
0.17 |
2.53 |
4.96 |
4.175 |
0.274 |
0.139 |
46.33 |
35.11 |
50.45 |
38.57 |
|
0.0025 |
0.16 |
2.23 |
4.53 |
4.331 |
0.311 |
0.15 |
47.69 |
36.68 |
||
|
0.0050 |
0.15 |
1.72 |
4.12 |
4.62 |
0.403 |
0.168 |
49.03 |
37.57 |
||
|
0.0075 |
0.14 |
1.49 |
3.83 |
4.95 |
0.465 |
0.181 |
49.73 |
37.61 |
||
|
0.0100 |
0.14 |
0.95 |
3.41 |
4.95 |
0.729 |
0.203 |
49.78 |
37.71 |
||
|
0.0125 |
0.13 |
0.90 |
3.06 |
5.33 |
0.77 |
0.226 |
54.89 |
37.96 |
||
|
0.0250 |
0.12 |
0.99 |
2.73 |
5.78 |
0.70 |
0.254 |
55.73 |
47.38 |
||
The above said phytochemical constituents present in Garlic having many active center such as sulfer,oxygen and nitrogen,which are regarded as centers of adsorption.Where the results of temperatures study have shown that mechanical adsorption takes place through physical adsorption,this effect of the extract cannot be ascribed to a definite component.It was found that aluminum surface in acid media have a positive charge36,which leads to electrostatic attraction of the negatively charge species(in this case Cl– ions).In aqueous acidic solutions main constituents of Garlic exist either as neutral molecules or as protonated molecules(cations).So in view of the above the adsorption mechanism may occur as follows
- First,the acid’s anions(Cl–) adsorb physically on the positively charged metal surface,resulting surface charge modification significantly to negative charge.
- Second,the protonated molecules are physically attracted to the anions layer which is formed on the metal surface.
The degree of protection increases with increase in extract concentration due to higher degree of surface coverage resulting from enhanced inhibitor adsorption.
From Figure 10 reveal of the chemical structures of these phytochemical constituents reveal that these compounds are easily hydrolysable and the compounds can be adsorbed on the metal surface via the lone pair of electrons present on their oxygen which make a barrier for charge and mass transfer leading to decrease the interaction of the metal with the corrosive environment. As a result, the corrosion rate of the metal was decreased. The formation of film layer essentially blocks the discharge of H+ and dissolution of the metal ions. Due to electrostatic interaction, the protonated constituent‟s molecules are adsorbed (physisorption) and high inhibition is expected.
Conclusion
- The acid extract of Garlic acts as good and efficient inhibitor for the corrosion of aluminum in hydrochloric acid medium.
- Inhibition efficiency increases with the increase of extract concentration and decreases with rise in temperature .
- The adsorption of different concentrations of the plant extract on the surface of the aluminum in 0.5 M HCl acid followed both Langmuir and Temkin adsorption isotherm.
- The values of standard free energy of adsorption suggest that the adsorption of inhibitor on aluminium surface occurred by physisorption mechanism. the negative sign of the Free Energy of adsorption indicates that the adsorption of the inhibitors on the aluminum surface was a spontaneous process.
- The negative values of enthalpy of adsorption (ΔH) suggest that the chemical reaction involved in the adsorption of the inhibitors on the metal surface is an exothermic process, hence increase in the reaction temperature of the medium will decrease the inhibition efficiency.
- The negative values of entropy of adsorption indicate that the reaction was spontaneous and feasible.
- Mechanism of physical adsorption is proposed and a first order type of reaction is obtained from the kinetic treatment of the data.
REFERENCES
- Ghali, E,John Wiley & Sons, Inc.New Jersey .2010,3.
- United States Army Corps of Engineers. EM 1110-2-1614 Design of Seawalls and Bulkheads. Washington DC:USACE. 1995
- Abdallah ,M. Corros Sci.2004,46 ,981-96.
- Sherifa ,E,M .; Su-Moon ,P. J Electrochm Soc .2005 ,152,6,B205-B211.
- Rehim, S. S. A.; Hassan, H. H. ; Amin, M. A. Appl. Surf. Sci. 2002 ,187 ,279-290.
- Brett ,C.M.A. Corros Sci. 1992, 33, 203-210.
- Ambat, R.; Dwarakodasa, E.S. J. Appl. Electrochem. 1994, 24, 911.
- Sato, N. Corros Sci .1995, 37 ,12, 1947-1967.
- Badawy, W.A.;Al-Kharafi, F.M .; El-Azab ,A.A. Corros Sci. 1999, 41,709-727
- Yazdzad, A.R.;Shahrabi ,T .;Hosseini ,M.G. Mater. Chem. Phys .2008,109, 199-205.
- Yildirim ,A .; Cetin ,M. Corros Sci .2008,50,1, 155-165.
- Noor, E.A. Matter. Chem.Phys. 2009, 114, 533-41. Abiola, O.K.; Oforka, N.C.; Ebenso, E.E.; Nwinuka, N.M. Anti-Corros Methods Materials. 2007 , 54, 219-224
- Kliskic, M.; Radoservic, J.; Gudic, S .;Katalinic, V. J Appl. Electrochem .2000, 30, 7, 823–830.
- El-Etre ,A.Y.Corros Sci .1998, 40, 1845–1850.
- El-Etre ,A. Y. Corros Sci .2003, 45 , 11,2485–2495.
- James, A.O .;Akaranta, O. African Journal of Pure and Applied Chemistry. 2009, 3, 262-268.
- Abiola, O.K.; Tobun, Y. Chinese Chem Lett .2010, 21,1449-1452.
- Li,X.Li.; Deng, S. Corros Sci. 2012,65,299 -308
- Yadav,S;Choudhary, G; Sharma ,A . International Journal of ChemTech Research. 2013,5, 4,1815-1823
- Corrosion Basics, “An Introduction”, National Association of Corrosion Engineers (NACE)(1984) 34.
- Jones, D.A. “Principles and Prevention of Corrosion”, 2nd Edition, Printice-Hall Inc., Upper Saddle River, NJ USA(1996) 31-34.
- Ebenso, E.E.; Ibok, U.J.; Ekpe, U.J.; Umoren ,S.; Jackson, E .; Abiola ,O.K .; Oforka ,N.C .; Martinez, S. Transactions of Saest (Cecri, India). 2004,39,4, 117 – 123
- Ita, B.I.; Offiong, O.E. Mater. Chem. Phys. 1997, 48, 164 –169.
- Okafor ,P.C.; Ebenso, E.E.; Ekpe U.J. Bull.Chem. Soc. Ethiopia .2004, 18 , 181 – 192.
- Buchweishaiia,J. Tanz,J.Sci .2003,29,99-108
- Quraishi, M.A.; Yadav, D.K.;. Ahamad ,I. Open Corrosion Journal. 2009, 2,56-60.
- Umoren, A.U.; Obot I.B.; Ebenso E.E.; Oxafor P.C. Portugaliae Electrochimica Acta .2008,26,267-282.
- Popova ,A.; Sokolova, E.; Raicheva,S.; Christov,M. Corros Sci .2003,45,33-35
- Shreir ,L.L.,Corrosion,Volume2,2nd.,Newnes-butterworths,London,1977.
- Likhanova, N.V.; Dominguez-Aguilar, M.A.; Olivares-Xometl, O.; Nava-Entzana, N; Arce, E.; Dorantes H.Corros Sci .2010,52,6و2088-2097
- Wabanne, J.T .; Okafor, V. Journal of Emerging Trends in Engineering and Applied Sciences.2001,2,4 , 619-62.
- James ,A.O.; Oforka, N.C.; Abiola ,O.K.Bull Electrochem .2006,22,3, 111-116
- Ismail, M.; Abdulrahman, A.S .;nd Hussain ,M.S. Int J Eng Sci Tech .2011, 3 ,1742-1748.
- Blaedel ,W.J .; Meloche , V.W.,“Elementary Quantitative Analysis; Theory and Practice, Second Edition, Harper & Row,Publishers, Incorporated, 49 East 33rd Street, New York 16, New York. (1963) 684-698.
- Tschapek, M.; Wasowski, C.; Torres sanche ,R.M. J.Electroanal.Chem .1976,47,167-176

This work is licensed under a Creative Commons Attribution 4.0 International License.









